Transdisciplinary Science and Engineering News
Isomerism Can Control and Increase the Diversity of Structure of Covalent Organic Frameworks, Emerging Nanoporous Solids
Researchers at Tokyo Institute of Technology for the first time discovered the selective generation of three types of structural isomers (a set of different nanostructures with an identical chemical composition) of three-dimensional covalent organic frameworks (3D-COFs), emerging nanoporous solids proposed for many applications, creating new freedom for structural and property controls of 3D-COFs.
Porous solids play a myriad of roles in the world. Examples include activated carbon, zeolite, and metal-organic frameworks (MOFs). Solid electrolytes in next-generation batteries have ion migration channels and hence are porous solids in a broad sense. Because the properties of porous solids are dictated by the pore size, the way the pores are connected internally, and the chemical nature of the pore walls, achieving high degrees of freedom in designing these properties is crucial.
As a novel class of nanoporous solids, COFs are formed by covalently and repeatedly condensing building block molecules. COFs have recently been extensively studied because they offer high design freedom in terms of function and microscopic geometries in the materials, as well as the high thermal stability that is desired for applications. Especially, as a subclass of COFs, three-dimensional COFs (3D-COFs) are expected to be useful owing to their inherently richer framework topologies than that in preceding two-dimensional COFs; most of the previous COF studies were for 2D-COFs.
However, there has been a dilemma. COFs have more stable and harder covalent bonds than MOFs, which have weaker and softer coordination bonds. This results in an advantage and two disadvantages. An advantage is the higher stability that enhances the durability during use. The first disadvantage is the poorer topological diversity in the framework geometries so far achieved. The second disadvantage is the difficulty in obtaining COFs with high crystallinity to the extent that crystal shapes are recognizable using an optical microscope. Both these disadvantages arise from the same root—the highly stiff and directional nature of covalent bonds (as compared to less stiff and less directional coordination bonds constituting MOFs). Addressing these disadvantages has been inevitable to boost applications of 3D-COFs.
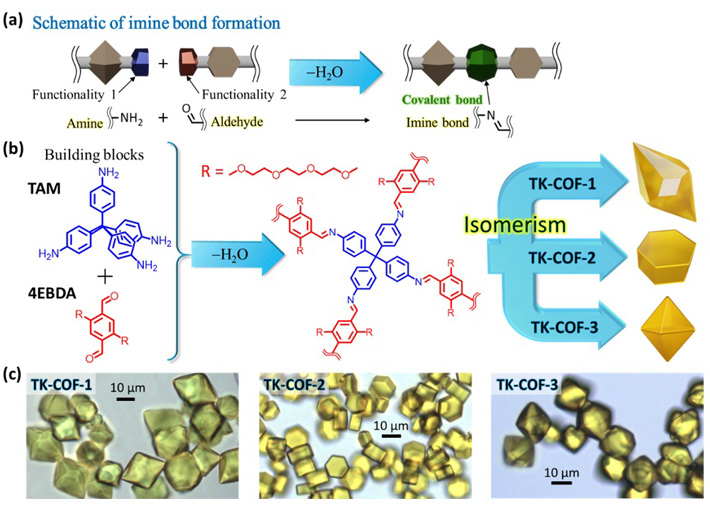
To resolve these issues, a research team led by Professor Yoichi Murakami of the Laboratory for Zero-Carbon Energy at the Institute of Innovative Research, Tokyo Institute of Technology attempted to create a new type of 3D-COFs by combining building block molecules that contain flexible moieties. As shown by Figures 1a and b, they selected TAM, which is a monomer with angular flexibility in the directions of the four amine functionalities, and 4EBDA, which is a monomer with two flexible side chains and two aldehyde functionalities, to condense them by forming imine bonds—one of the well-known covalent bonds in organic compounds. The sidechain ("R" in Figure 1b) is a part of polyethylene glycol, which is a functionality known to enhance the transport of Lithium ions and is therefore usable as a solid-state electrolyte in Lithium-ion batteries.
Significantly, after repeated trials of many formation conditions in solution, the research group produced beautiful, high-quality crystals with three distinctly different shapes shown in Figure 1c. The researchers named these crystals TK-COF-1, TK-COF-2, and TK-COF-3. Surprisingly, the research team discovered that these three new COFs had an identical chemical composition.
- Figure 1.
- 3D-COFs that exhibit framework isomerism discovered in this work. (a) Schematic of imine bond formation. (b) The building block molecules chosen in this work (R: polyethylene glycol chain) and the construction of an extended network by imine bond formations through polycondensation of the molecules. (c) Optical micrographs of TK-COF-1, TK-COF-2, and TK-COF-3 for the first time grown in this study.
- Figure 1.
- 3D-COFs that exhibit framework isomerism discovered in this work. (a) Schematic of imine bond formation. (b) The building block molecules chosen in this work (R: polyethylene glycol chain) and the construction of an extended network by imine bond formations through polycondensation of the molecules. (c) Optical micrographs of TK-COF-1, TK-COF-2, and TK-COF-3 for the first time grown in this study.
The X-ray diffraction measurements of the crystals revealed their qualitatively different nanostructures as shown in Figures 2a to c. The framework topologies of these crystals were found to be dia (TK-COF-1), qtz (TK-COF-2), and dia-c3 (TK-COF-3). This is an important finding that the diversity of structures and properties of 3D-COFs can be increased not only by the conventional way of choosing block molecules but also by controlling the emergence of isomer type during polycondensation of building block molecules. The density of COFs (indicated in the figure panels) was successfully changed by a factor of about 3 due to the choice of isomers, as indicated in the panels of Figure 2.
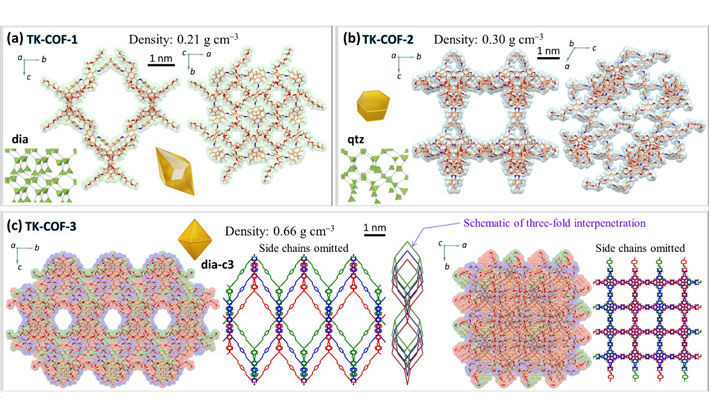
- Figure 2.
- Microscopic framework structures of (a) TK-COF-1, (b) TK-COF-2, and (c) TK-COF-3. These were found to have dia, qtz, and dia-c3 framework topologies, respectively.
In summary, this work demonstrates that key properties of 3D-COFs (density, pore size, mechanical properties, etc.) can be tuned with a new degree of freedom utilizing the emergence and control of structural isomerism. This outcome will accelerate the future applications of COFs.
The original paper was published online January 11, 2024 from Journal of the American Chemical Society![]() .
.
- Reference
| Authors : | X. Wang1,2, Y. Wada3, T. Shimada3, A. Kosaka4, K. Adachi5, D. Hashizume5, K. Yazawa6, H. Uekusa3, Y. Shoji4, T. Fukushima4, M. Kawano3, and Y. Murakami1,2 |
|---|---|
| Title : | Triple Isomerism in 3D Covalent Organic Frameworks |
| Journal : | Journal of the American Chemical Society |
| DOI : | 10.1021/jacs.3c13863 |
| Affiliations : |
1Laboratory for Zero-Carbon Energy, Institute of Innovative Research, Tokyo Institute of Technology, Japan 2Department of Mechanical Engineering, School of Engineering, Tokyo Institute of Technology, Japan 3Department of Chemistry, School of Science, Tokyo Institute of Technology, Japan 4Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, Japan 5RIKEN Center for Emergent Matter Science (CEMS), Japan 6JEOL Ltd., Japan |
- Improving the Properties of Sweeteners for Enhanced Thermal Energy Storage | Tokyo Tech News
- Solid material that 'upconverts' visible light photons to UV light photons could change how we utilize sunlight | Tokyo Tech News
- Doing Photon Upconversion a Solid—Crystals that Convert Light to More Useful Wavelengths | Tokyo Tech News
- Thermo-chemical power generation integrated with forced convection cooling to create a self-sustaining liquid cooling system | Tokyo Tech News
- Sustainable solvent platform for photon upconversion increases solar utilization efficiency | Tokyo Tech News
- MIC "INNO-vation" Generation Special Corporate Award for Associate Professor Yoichi Murakami | Tokyo Tech News
- Yoichi Murakami | Researcher Finder - Tokyo Tech STAR Search
- Masaki Kawano | Researcher Finder - Tokyo Tech STAR Search
- Takanori Fukushima | Researcher Finder - Tokyo Tech STAR Search
- Yoshiaki Shoji | Researcher Finder - Tokyo Tech STAR Search
- Hidehiro Uekusa | Researcher Finder - Tokyo Tech STAR Search
- Murakami Laboratory
- Kawano Laboratory
- Fukushima Laboratory
- Uekusa Laboratory (Japanese)
- Laboratory for Zero-Carbon Energy
- Institute of Innovative Research (IIR)
- Global Engineering for Development, Environment and Society Graduate Major|Education|Department of Transdisciplinary Science and Engineering, School of Environment and Society
- Nuclear Engineering Graduate Major|Education|Department of Transdisciplinary Science and Engineering, School of Environment and Society
- Transdisciplinary Science and Engineering Undergraduate Major|Education|Department of Transdisciplinary Science and Engineering, School of Environment and Society
- Department of Mechanical Engineering, School of Engineering
- Department of Chemistry, School of Science
- Laboratory for Chemistry and Life Science
- Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Latest Research News
School of Environment and Society
—Creating Science and Technology for Sustainable Environment and Society—
Information on School of Environment and Society inaugurated in April 2016
School of Engineering
—Creating New Industries and Advancing Civilization—
Information on School of Engineering inaugurated in April 2016
School of Science —Exploring the Truth and Creating Knowledge—
Information on School of Science inaugurated in April 2016
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Professor Yoichi Murakami
Institute of Innovative Research, Tokyo Institute of Technology
Email murakami.y.af@m.titech.ac.jp
Tel +81-3-5734-3836