Life Science and Technology News
Revealing the molecular structures of sugars using galectin-10 protein crystals
Researchers develop a rapid cell-free crystal-based method for gaining detailed insights on the structure and dynamics of sugars
A quick, purification-free method was developed by researchers at Institute of Science Tokyo, to capture the detailed 3D structures of flexible sugar molecules. By growing crystals of galectin-10 protein using a cell-free crystallization process and soaking them in sugar solution, the researchers were able to trap and analyze the molecular arrangement of sugars and their interactions with the protein. This offers a powerful tool to accelerate research in drug discovery and molecular biology.
Rapid Structural Analysis of Flexible Sugars with Galectin-10 (Gal-10) Crystals
Cell-Free Protein
Crystallization Enables Rapid Structure Determination of Disaccharides and Trisaccharides Using Galectin-10
Crystals![]()
Kojima et al. (2025) | Small Structures
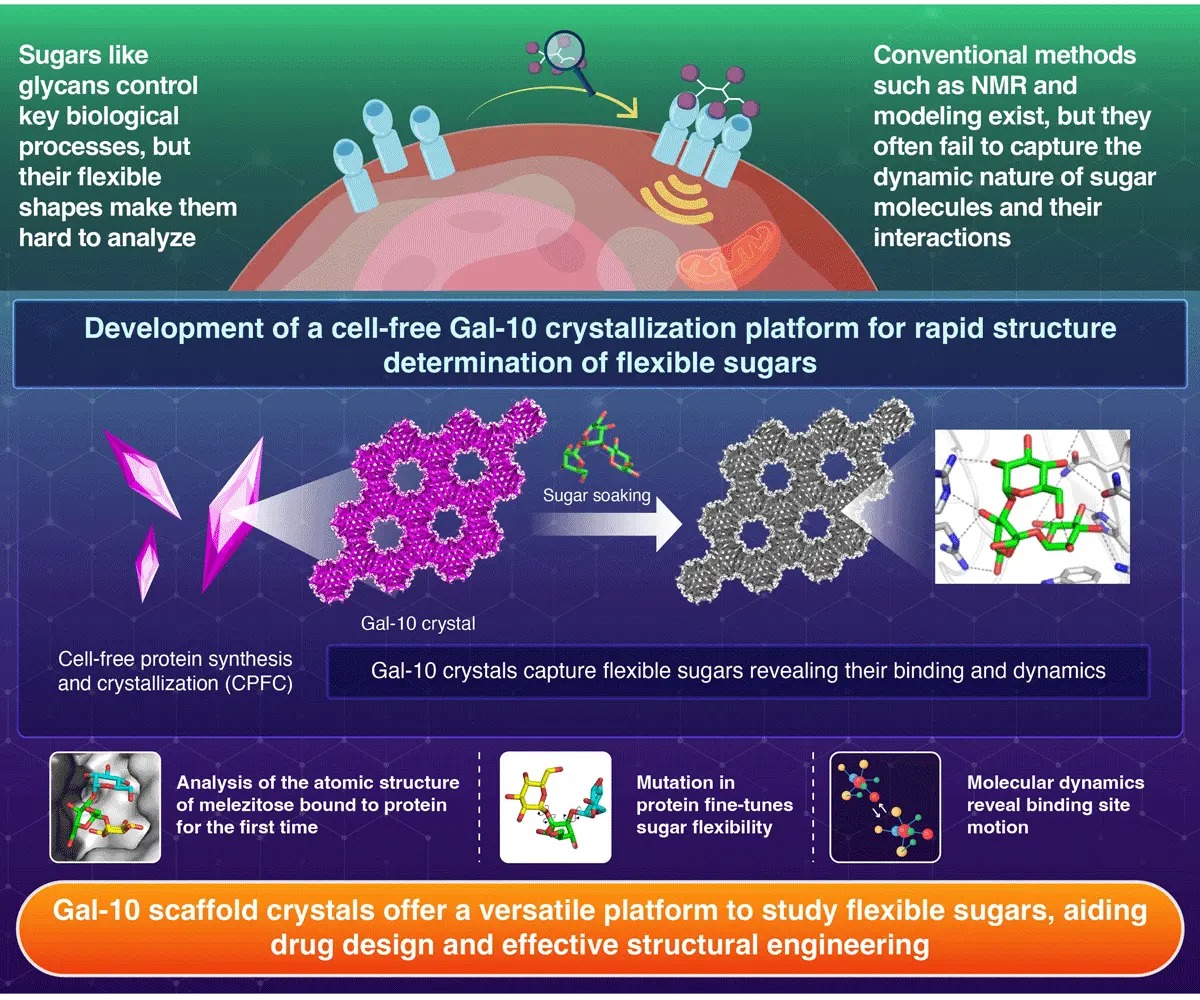
Sugars, or saccharides, do much more than sweeten food. In living organisms, these molecules decorate the surfaces
of cell and also act as vital messengers in processes such as infection control and tissue repair. Understanding how
these sugar molecules fold, move, and interact with proteins is important for interpreting their roles. But
structural complexity and flexibility make them difficult to study using conventional techniques.
To overcome this challenge, a research team at Institute of Science Tokyo(Science Tokyo), Japan, led by Professor
Takafumi Ueno from the School of Life Science and Technology developed a rapid, purification-free method for
structural analysis of sugars. Their study was
published in the journal Small Structures![]() on October 23, 2025.
on October 23, 2025.
Using an innovative cell-free protein crystallization (CFPC) system, the team synthesized crystals of a
sugar-binding protein called galectin-10 (Gal-10). This process simply involved crystallization within a test-tube
without the need of a cell culture. Within just a day, these protein crystals formed molecular structures that could
trap sugar molecules.
“We developed scaffold-like crystals of Gal-10 that hold sugar molecules within their network, allowing us to
visualize the precise conformations of sugars at the atomic level using X-ray techniques,” explains Ueno.
Once the Gal-10 crystals were developed, the team incorporated several natural sugars into these crystals by soaking
in sugar solutions. In this way, the team successfully captured the detailed 3D structures of five different sugars
including the first ever atomic-resolution image of melezitose, a complex sugar previously too flexible to study in
detail. They also mapped raffinose, a prebiotic sugar which is known to be beneficial for gut bacteria.
Furthermore, the researchers also analyzed the effect of protein mutation on the change in binding of sugar.
Replacing a single amino acid (E33A) in the Gal-10 protein allowed the sugar molecules to fit more snugly within the
crystal, revealing how small protein modifications influence sugar binding.
“Being able to visualize these sugars so rapidly gives us an entirely new way to explore their behavior,” says Ueno.
“We can now observe directly how sugar molecules move and interact with proteins which is the key to understanding
many biological processes.”
To gain a deeper insight into the molecular behavior of the sugars, the team combined X-ray crystallography
technique with molecular dynamics simulations. This allowed them to track how the sugars moved over time. The
analyses revealed that the crystal gently restricts sugar motion, thus stabilizing them for detailed imaging.
Additionally, the approach also showed how tiny shifts in the molecular shapes of sugars affect binding strength,
providing details that could aid design of new drugs and biomolecules.
According to the researchers, this CFPC–Gal-10 platform opens the door to faster, more precise analysis of complex
carbohydrates. “With our system, researchers could screen hundreds of sugars and small molecules within a short time
span, accelerating discoveries in glycobiology, drug development and biomaterials,” concludes Ueno.
Reference
| Authors: | Mariko Kojima1, Xinchen Yao1, Satoshi Abe1, Tadaomi Furuta1, Kunio Hirata2, Ririko Kobayashi1, Taiga Suzuki1, and Takafumi Ueno1,3,* |
|---|---|
| Title: | Cell-Free Protein Crystallization Enables Rapid Structure Determination of Disaccharides and Trisaccharides Using Galectin-10 Crystals |
| Journal: | Small Structures |
| DOI: | 10.1002/sstr.202500501 |
| Affiliations: | 1School of Life Science and Technology, Institute of Science Tokyo, Japan 2SR Life Science Instrumentation Unit, RIKEN/SPring-8 Center, Hyogo, Japan 3International Research Frontiers Initiative (IRFI), Institute of Science Tokyo, Japan |
| *Corresponding author’s email: | ueno.t.bb33@m.isct.ac.jp |
Related articles
- Designing efficient artificial enzymes with self-assembling protein cages | Life Science and Technology News
- Controlling Conformational Changes in Protein Aromatic Side Chains | Life Science and Technology News
- Visualizing Short-Lived Intermediate Compounds Produced During Chemical Reactions | Life Science and Technology News
- Developing a System to Study Proteins Without Fixed Structures | Life Science and Technology News
- Towards Artificial Photosynthesis with Engineering of Protein Crystals in Bacteria | Life Science and Technology News
- Novel Cell-Free Protein Crystallization Method to Advance Structural Biology | Life Science and Technology News
- Decoding Protein Assembly Dynamics with Artificial Protein Needles | Life Science and Technology News
- In-cell Nano-3D Printer: Synthesizing Stable Filaments from In-cell Protein Crystals | Life Science and Technology News
- Nanotubes built from protein crystals: Breakthrough in biomolecular engineering | Life Science and Technology News
- Takafumi Ueno | Science Tokyo Research Information DB - Science and engineering fields
- Tadaomi Furuta | Science Tokyo Research Information DB - Science and engineering fields
- Ueno Laboratory
- Department of Life Science and Technology, School of Life Science and Technology
- School of Life Science and Technology
- Laboratory for Chemistry and Life Science, Institute of Integrated Research | Institute of Science Tokyo
- Institute of Integrated Research
Further Information
Professor Takafumi Ueno
School of Life Science and Technology, Institute of Science Tokyo
Email ueno.t.bb33@m.isct.ac.jp