Life Science and Technology News
Controlling Conformational Changes in Protein Aromatic Side Chains
Scientists develop a protein cage system that enables coordinated molecular reorientations in proteins, with potential applications in biotechnology
Novel protein cage system can control and visualize orientational changes in aromatic side chains upon ligand binding, as reported by researchers at Institute of Science Tokyo. By inducing coordinated molecular changes, this approach enables precise control over protein dynamics while also enhancing fluorescence properties. Their breakthrough could lead to applications in biomolecular robotics, drug delivery, and advancing the development of responsive biomaterials.
Ligand-Induced Aromatic Cluster Dynamics for Tunable Fluorescence in Protein Cages
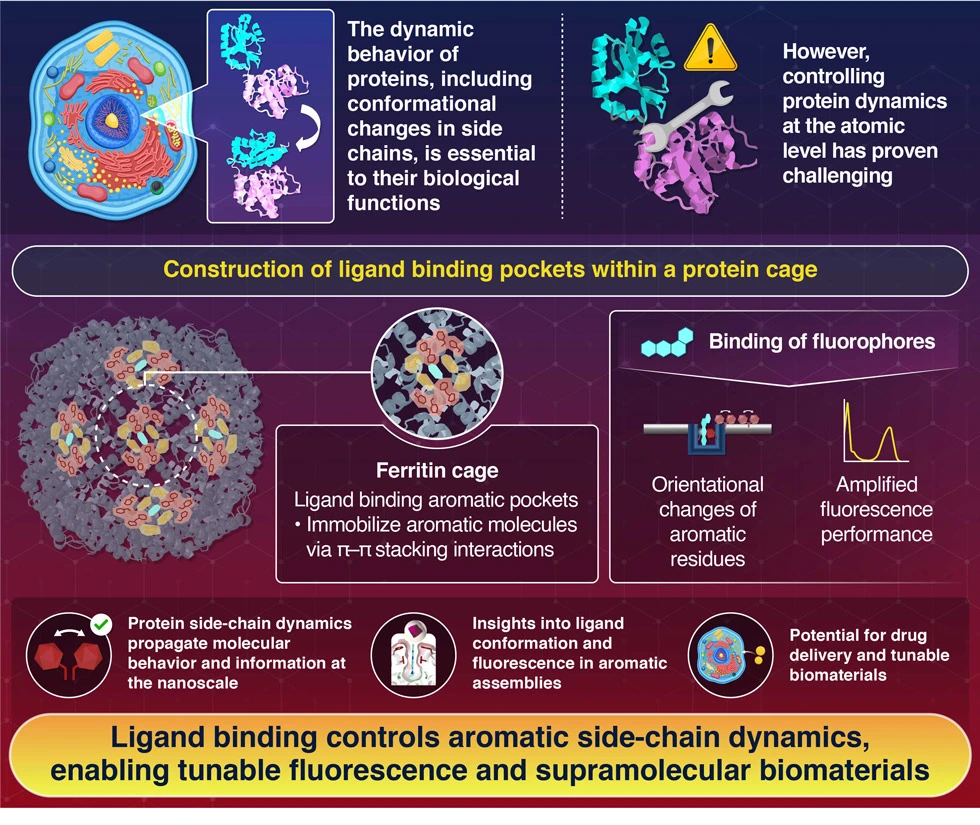
Design of Aromatic Interaction Networks in a Protein Cage Modulated by Fluorescent Ligand Binding
![]()
Hishikawa et al. (2025) | Advanced Science
The dynamic nature of proteins—their ability to bend, fold, and change shape in response to their environment—underlies most aspects of cellular function. Conformational changes in proteins, which occur at the nanoscale, are fundamental to processes ranging from enzyme catalysis and chemical signaling to cell division and differentiation.
Aromatic interactions are particularly important in protein dynamics, playing roles in protein folding, molecular recognition, and the stabilization of protein complexes. However, designing systems that can control protein dynamics at an atomic level, such as the orientation of specific aromatic side chains, has remained a significant challenge in applied chemistry and biomaterials. In turn, this has limited our ability to develop sophisticated protein-based technologies.
Fortunately, a research team led by Professor Takafumi Ueno from Institute of Science Tokyo (Science Tokyo), Japan, has been working on innovative solutions towards this goal. In their latest study, published in Advanced Science![]() on 20 February 2025, the researchers designed a protein cage system that can control and visualize the movement of multiple aromatic side chains through the strategic binding of fluorescent ligands. Their approach represents a significant breakthrough in protein engineering, offering unprecedented control over certain protein dynamics.
on 20 February 2025, the researchers designed a protein cage system that can control and visualize the movement of multiple aromatic side chains through the strategic binding of fluorescent ligands. Their approach represents a significant breakthrough in protein engineering, offering unprecedented control over certain protein dynamics.
The team engineered special “pockets” within a protein cage structure called ferritin, surrounding these pockets with carefully arranged clusters of aromatic amino acids. When specific fluorescent ligands bind to these pockets, they trigger what could be described as a molecular domino effect—a coordinated series of changes in the orientation of the surrounding aromatic side chains.
Through detailed X-ray crystallography analysis, the researchers demonstrated that different ligand structures could produce different orientational changes in the aromatic side chain. The team systematically investigated this phenomenon by designing variants with different numbers and types of phenylalanine group substitutions. This approach allowed them to fine-tune the system’s response to different molecular binding events. Interestingly, when certain fluorescent molecules were isolated within these aromatic pockets, they also exhibited markedly enhanced fluorescence properties, including improved quantum yield and longer fluorescence lifetime. “Together, these findings provide an understanding of the unique molecular behavior and fluorescence properties of ligands due to the assembly of aromatic residues, and a guideline for developing dynamically controlled supramolecular biomaterials,” explains Ueno.
The success of this approach hinged on the team’s innovative strategy of using ligand binding as a driving force to induce and propagate changes in aromatic side chains. Manipulating protein side-chain dynamics could be used to propagate molecular behavior and information across nanoscale distances, which could prove useful in a number of ways. “The expected applications of this technology include the creation of biomolecular robots responsive to external stimuli and the use of such protein systems for drug delivery, especially for compounds with poor solubility in water,” notes Ueno. The system’s ability to enhance fluorescence properties while providing controlled molecular movement could also prove particularly valuable for biosensors.
Overall, this study opens new possibilities in medicine, biotechnology, and materials science, paving the way to responsive biomaterials that can be precisely controlled at the molecular level.
- Reference
| Authors: |
Yuki Hishikawa1, Taiga Suzuki1, Basudev Maity1, Hiroki Noya1, Michito Yoshizawa2, Asuka Asanuma1, Yuri Katagiri2, Satoshi Abe1, Satoru Nagatoishi3, Kouhei Tsumoto3,4, Takafumi Ueno1,5,*
*Corresponding author |
|---|---|
| Title: | Design of Aromatic Interaction Networks in a Protein Cage Modulated by Fluorescent Ligand Binding |
| Journal: | Advanced Science |
| DOI: |
10.1002/advs.202417030 |
| Affiliations: |
1School of Life Science and Technology, Institute of Science Tokyo, Japan
2Laboratory for Chemistry and Life Science, Institute of Integrated Research, Institute of Science Tokyo, Japan 3The Institute of Medical Science, The University of Tokyo, Japan 4School of Engineering, The University of Tokyo, Japan 5Research Center for Autonomous Systems Materialogy (ASMat), Institute of Integrated Research, Institute of Science Tokyo, Japan |
Related articles
- Developing a System to Study Proteins Without Fixed Structures | Life Science and Technology News
- Visualizing Short-Lived Intermediate Compounds Produced During Chemical Reactions | Life Science and Technology News
- Engineering Bacteria to Biosynthesize Intricate Protein Complexes | Life Science and Technology News
- Towards Artificial Photosynthesis with Engineering of Protein Crystals in Bacteria | Life Science and Technology News
- Novel Cell-Free Protein Crystallization Method to Advance Structural Biology | Life Science and Technology News
- Decoding Protein Assembly Dynamics with Artificial Protein Needles | Life Science and Technology News
- In-cell Nano-3D Printer: Synthesizing Stable Filaments from In-cell Protein Crystals | Life Science and Technology News
- Nanotubes built from protein crystals: Breakthrough in biomolecular engineering | Life Science and Technology News
- In cell molecular sieve from protein crystal | Life Science and Technology News
- Ferritin releases carbon monoxide in regulated therapeutic doses | Former Tokyo Tech
- Protein-engineered cages aid studies of cell functions | Former Tokyo Tech
- Takafumi Ueno | Researcher Finder - Science Tokyo STAR Search
- Michito Yoshizawa | Researcher Finder - Science Tokyo STAR Search
- Ueno Laboratory
- Yoshizawa & Sawada Laboratory
- Department of Life Science and Technology, School of Life Science and Technology
- Research Center for Autonomous Systems Materialogy (ASMat), Institute of Integrated Research
- Laboratory for Chemistry and Life Science, Institute of Integrated Research
- School of Life Science and Technology
- Institute of Integrated Research
Further Information
Professor Takafumi Ueno
School of Life Science and Technology, Institute of Science Tokyo
Email tueno@bio.titech.ac.jp





