Life Science and Technology News
Designing efficient artificial enzymes with self-assembling protein cages
Spatially arranged histidine residues drive efficient oxidation reactions in engineered ferritin structures
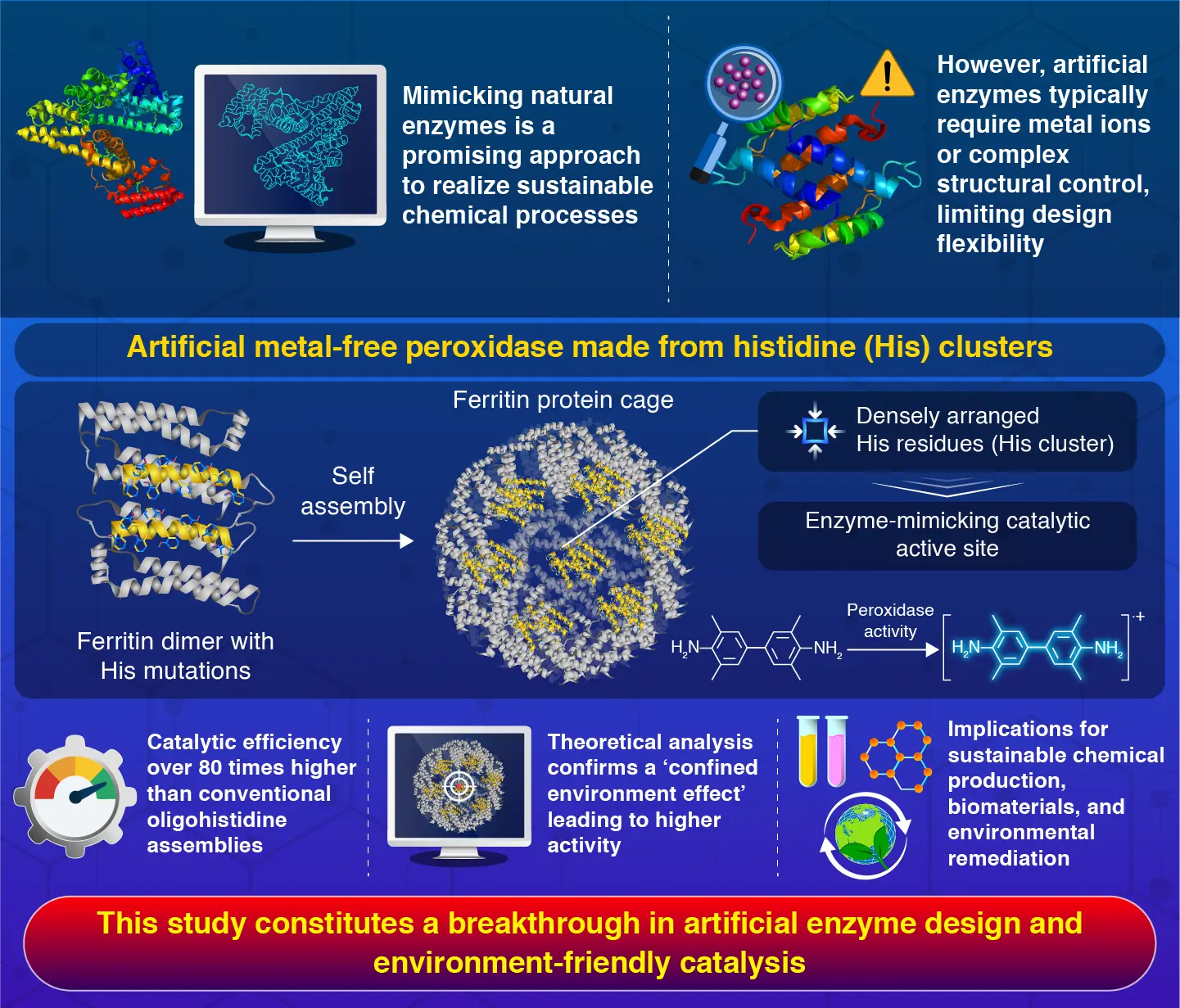
Strategically arranging histidine residues inside a protein cage is a promising approach to create artificial enzymes, reports researchers from Institute of Science Tokyo. The engineered protein cage mimics natural enzymes using simple amino acids without any metal cofactors, overcoming a major limitation in artificial enzyme design. Molecular simulations confirmed that the confined environment within the protein cage enhances catalytic efficiency, offering a new route to develop sustainable biocatalysts.
Creating Highly Active Metal-Free Enzymes
An Artificial Metal-Free Peroxidase Designed Using a Ferritin Cage for Bioinspired Catalysis
![]()
Tian et al. (2025) | Angewandte Chemie | 10.1002/anie.202504608
Natural enzymes are remarkable molecular machines that enable all sorts of essential biochemical reactions. For decades, scientists have sought to create artificial versions of these catalysts for industrial and biomedical applications. However, they have struggled to match nature’s efficiency and simplicity. This, in turn, has hindered the development of environmentally friendly catalysts for sustainable chemistry.
Creating artificial enzymes typically requires either cofactors or complex structural arrangements that precisely position reactive groups in three-dimensional space. These requirements constrain design flexibility, often resulting in enzymes that underperform compared to their natural counterparts. Finding simpler approaches that don’t sacrifice catalytic power has remained an elusive goal in the field of biocatalysis.
Against this backdrop, a research team led by Professor Takafumi Ueno from Institute of Science Tokyo, Japan, reported a novel approach to enzyme design using protein nanocages. Their paper, published in Angewandte Chemie![]() on April 24, 2025, demonstrates how precisely arranged histidine amino acids inside a ferritin protein cage can function as a highly effective metal-free peroxidase—an enzyme that drives oxidation reactions using hydrogen peroxide as a reactant.
on April 24, 2025, demonstrates how precisely arranged histidine amino acids inside a ferritin protein cage can function as a highly effective metal-free peroxidase—an enzyme that drives oxidation reactions using hydrogen peroxide as a reactant.
The researchers engineered the ferritin cage by introducing histidine residues and a series of targeted mutations. By taking advantage of ferritin’s ability to self-assemble into protein cages, they created clusters of histidine residues on the cage’s inner surface. These histidine clusters act as catalytic centers, mimicking peroxidase activity that promotes reactions between hydrogen peroxide and 3,3’,5,5’-tetramethylbenzidine substrate. “The engineered ferritin variant showed approximately 80 times higher reaction efficiency compared to conventional oligohistidine assemblies,” remarks Ueno.
The team’s innovative approach demonstrates that the proper spatial arrangement of simple amino acids can eliminate the need for metal cofactors in certain enzymatic reactions. Through careful positioning of these amino acids at the interfaces of the ferritin cage, the team produced a confined reaction environment that significantly enhanced catalytic activity. Using molecular dynamics simulations, they revealed how the ferritin cage confines reactants in close proximity to the histidine clusters, explaining the dramatic enhancement in catalytic efficiency. “Based on theoretical calculations, we confirmed that this high activity is further enhanced by a 'confined environment effect' within the protein cage, which concentrates reactants and facilitates their interaction,” says Ueno.
These exciting findings unlock new possibilities for protein cages in metal-free catalytic systems, which could find applications in sustainable chemical production, biomaterials development, and environmental remediation. “This research represents a major advancement in artificial enzyme design and environmentally friendly catalysis, paving the way for the development of sustainable biocatalysts,” concludes Ueno.
In the near future, further studies in this field could lead to high-performance bioinspired catalysts.
- Reference
| Authors: |
Jiaxin Tian1, Basudev Maity1, Tadaomi Furuta1, Tiezheng Pan1, and Takafumi Ueno1,2*
*Corresponding author |
|---|---|
| Title: | An Artificial Metal-Free Peroxidase Designed Using a Ferritin Cage for Bioinspired Catalysis |
| Journal: | Angewandte Chemie |
| DOI: |
10.1002/anie.202504608 |
| Affiliations: |
1School of Life Science and Technology, Institute of Science Tokyo, Japan
2Department of Life Science and Technology, Research Center for Autonomous Systems Materialogy (ASMat), Institute of Integrated Research, Institute of Science Tokyo, Japan |
Related articles
- Controlling Conformational Changes in Protein Aromatic Side Chains | Life Science and Technology News
- Visualizing Short-Lived Intermediate Compounds Produced During Chemical Reactions | Life Science and Technology News
- Developing a System to Study Proteins Without Fixed Structures | Life Science and Technology News
- Towards Artificial Photosynthesis with Engineering of Protein Crystals in Bacteria | Life Science and Technology News
- Novel Cell-Free Protein Crystallization Method to Advance Structural Biology | Life Science and Technology News
- Decoding Protein Assembly Dynamics with Artificial Protein Needles | Life Science and Technology News
- In-cell Nano-3D Printer: Synthesizing Stable Filaments from In-cell Protein Crystals | Life Science and Technology News
- Dynamic self-assembly of designed protein 'Needles' | YouTube
- Basudev Maity | Researcher Finder - Science Tokyo STAR Search
- Tadaomi Furuta | Researcher Finder - Science Tokyo STAR Search
- Takafumi Ueno | Researcher Finder - Science Tokyo STAR Search
- Ueno Lab.
- Department of Life Science and Technology, School of Life Science and Technology
- School of Life Science and Technology
- Laboratory for Chemistry and Life Science, Institute of Integrated Research
- Institute of Integrated Research
Further Information
Professor Takafumi Ueno
School of Life Science and Technology, Institute of Science Tokyo
Email ueno.t.bb33@m.isct.ac.jp





