Life Science and Technology News
Understanding the Regulation of Apicoplast Gene Expression in the Malaria Parasite
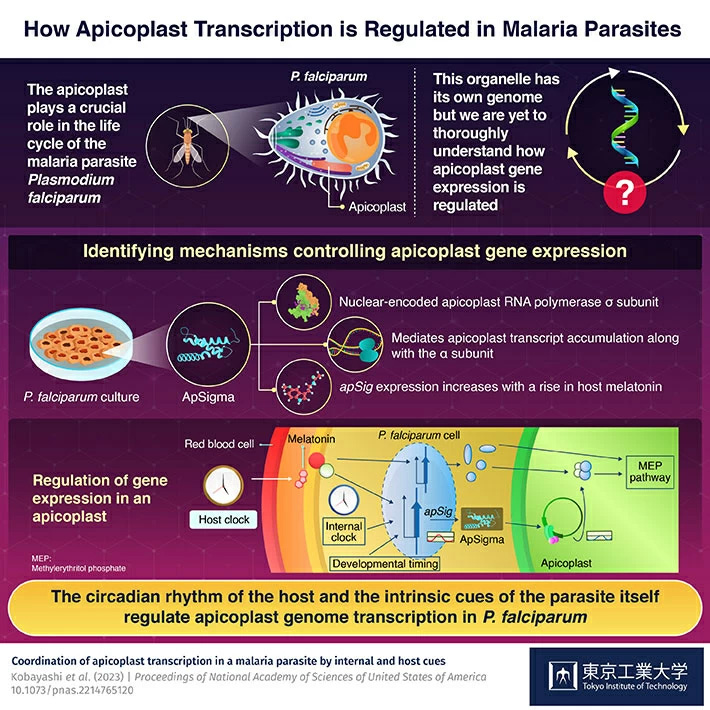
Gene expression within the apicoplast, an organelle in the malaria parasite Plasmodium falciparum, is regulated by melatonin (the circadian signaling hormone) in host blood, and intrinsic parasite cues, via a factor called ApSigma, as identified by a recent study aided by Tokyo Tech's World Research Hub Initiative. The regulatory system highlighted in this study might be a future target for malaria treatment.
Malaria is one of the biggest public health risks, with around 240 million people from across the globe contracting it every year. However, this life-threatening disease is not contagious. It is transmitted by the bite of a female Anopheles mosquito infected with the malaria parasite, Plasmodium falciparum (Figure 1)
. This parasite enters the human body through the mosquito bite and causes symptoms like fever, cold, fatigue, and headache, which are highly periodic. The periodicity of symptoms can be linked to the synchronization of the parasite's life cycle with the circadian rhythm—i.e., the 24-hour internal biological clock—of the infected person or the host.

Figure 1. A female Anopheles mosquito
- Malaria, transmitted by mosquito bites, is still a serious infectious disease that kills many people (image courtesy of Assistant Professor Kyoko Futami, Nagasaki University).
P. falciparum contains an apicoplast, a unique cellular organelle which contains its own genome and is crucial for the parasite's life cycle. Despite its importance, however, not much is known about the mechanisms regulating gene expression in apicoplasts and their potential role in modulating the observed periodicity of malaria symptoms, or the life cycle of P. falciparum. This is why recently, a team of scientists led by Professor Kan Tanaka, of Tokyo Institute of Technology (Tokyo Tech), undertook a joint research project to take a closer look at the underlying mechanisms that mediate apicoplast gene expression. The work, published in Proceedings of National Academy of Sciences of United States of America (PNAS)![]() , was a result of collaboration with co-authors Professor Kiyoshi Kita of Nagasaki University and Professor Antony N. Dodd, a group leader at the John Innes Centre in the UK
, was a result of collaboration with co-authors Professor Kiyoshi Kita of Nagasaki University and Professor Antony N. Dodd, a group leader at the John Innes Centre in the UK![]() —also a visiting professor at Tokyo Tech—facilitated by the institute's World Research Hub Initiative (WRHI), a project for interdisciplinary collaboration with leading researchers from across the world
—also a visiting professor at Tokyo Tech—facilitated by the institute's World Research Hub Initiative (WRHI), a project for interdisciplinary collaboration with leading researchers from across the world![]() .
.
"Previous studies have shown that certain plant σ subunits participate in the circadian regulation of gene expression in plastids (i.e., organelles like the apicoplast). Therefore, the present study hypothesized that a nuclear-encoded σ subunit might coordinate apicoplast gene expression with the life cycle of P. falciparum or the circadian rhythm of its host," explains Prof. Tanaka.
The team cultured P. falciparum in a lab and studied it using phylogenetic analysis and immunofluorescence microscopy techniques. As a result, they identified ApSigma, a nuclear encoded apicoplast RNA polymerase σ subunit. It, along with the α subunit, likely mediates apicoplast transcript accumulation, whose periodicity is akin to that of the parasite's developmental control. In addition, apicoplast transcription and expression of the apicoplast subunit gene, apSig, increased in the presence of melatonin, the circadian signaling hormone present in host blood.
Based on the data collected from different tests, the scientists suggest that there is an evolutionarily preserved regulatory system in which the host's circadian rhythm is integrated with the parasite's intrinsic cues. Together, they coordinate genome transcription in the apicoplast of P. falciparum. This work lays solid groundwork for further studies in the field aiming to comprehensively explain the regulatory mechanisms of Plasmodium's cell cycle.
In conclusion, Prof. Tanaka highlights the future implications of the present research. "Malaria kills hundreds of thousands of people across the world, every year. This study identifies a regulatory system that might be a future target for malaria treatment."
Professor Dodd, adds to that, "It is amazing that a process we identified in plants has led to the discovery of an equivalent mechanism in a globally important pathogen. The new protein and mechanism identified could present a new target for the development of drugs for the treatment and or prevention of malaria, in both humans and farm animals."
Professor Kita signs off on a positive note. "This research demonstrates the value of international and interdisciplinary collaboration, and the power of plant sciences and microbiology to drive unusual and novel discoveries that could be of considerable global benefit," he says.
Here's hoping for efficient treatment of malaria!
- Reference
| Authors : | Yuki Kobayashi1, Keisuke Komatsuya2,3, Sousuke Imamura1,4, Tomoyoshi Nozaki2, Yoh-ichi Watanabe2, Shigeharu Sato1,5,6,7, Antony N. Dodd8, Kiyoshi Kita2,7,9, and Kan Tanaka1 |
|---|---|
| Title : | Coordination of apicoplast transcription in a malaria parasite by internal and host cues |
| Journal : | Proceedings of National Academy of Sciences of United States of America (PNAS) |
| DOI : | 10.1073/pnas.2214765120 |
| Affiliations : | 1Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology 2Department of Biomedical Chemistry, Graduate School of Medicine, The University of Tokyo 3Laboratory of Biomembrane, Tokyo Metropolitan Institute of Medical Science 4Space Environment and Energy Laboratories, Nippon Telegraph and Telephone Corporation 5Department of Pathology and Microbiology, Faculty of Medicine and Health Sciences, Universiti Malaysia Sabah 6Borneo Medical and Health Research Centre, Faculty of Medicine and Health Sciences, Universiti Malaysia Sabah 7School of Tropical Medicine and Global Health, Nagasaki University 8Department of Cell and Developmental Biology, John Innes Centre 9Department of Host-Defense Biochemistry, Institute of Tropical Medicine, Nagasaki University |
- Circadian Control of Chloroplast Transcription by a Nuclear-Encoded Timing Signal | Science
- The parasite intraerythrocytic cycle and human circadian cycle are coupled during malaria infection | PNAS
- Scientists find a "switch" to increase starch accumulation in algae | Life Science and Technology News
- Harnessing energy from algae: Scientists identify enzyme that could help accelerate biofuel production
- Target of rapamycin: linking cytosolic and chloroplast ribosome biogenesis in plants | Life Science and Technology News
- Abscisic Acid Helps Red Algae Tolerate Salt Stress by Controlling Cell Cycle Initiation | Tokyo Tech News
- 【Labs spotlight】 Tanaka and Imamura Laboratory | Life Science and Technology News
- TANAKA -YOSHIDA Laboratory
- Kan Tanaka | Researcher Finder - Tokyo Tech STAR Search
- Laboratory for Chemistry and Life Science Institute of Innovative Research
- Institute of Innovative Research (IIR)
- Department of Life Science and Technology, School of Life Science and Technology
- Tokyo Metropolitan Institute of Medical Science
- Graduate School of Medicine and Faculty of Medicine, The University of Tokyo
- Universiti Malaysia Sabah
- Cell and Developmental Biology | John Innes Centre
- Nagasaki University School of Tropical Medicine and Global Health
- Latest Research News
Further Information
Professor Kan Tanaka
Institute of Innovative Research, Tokyo Institute of Technology
E-mail : kntanaka@res.titech.ac.jp