Life Science and Technology News
Understanding the role of linear ubiquitination in T-tubule biogenesis
Linear ubiquitination by LUBEL triggers Amph-mediated T-tubule biogenesis in Drosophila
Transverse tubules (T-tubules) play significant role in muscle contraction. However, the underlying mechanism of their formation is yet to be elucidated. In a recent study, a research team from Japan used a Drosophila model to understand this process. The results show the involvement of LUBEL, an E3 ubiquitin ligase, in the T-tubule biogenesis. Beyond LUBEL’s role in immune response, the study reveals an unexpected function of linear ubiquitination in membrane deformation, driven by BAR-domain proteins.
Linear Ubiquitination in Transverse tubule (T-tubule) Formation and Membrane Remodeling
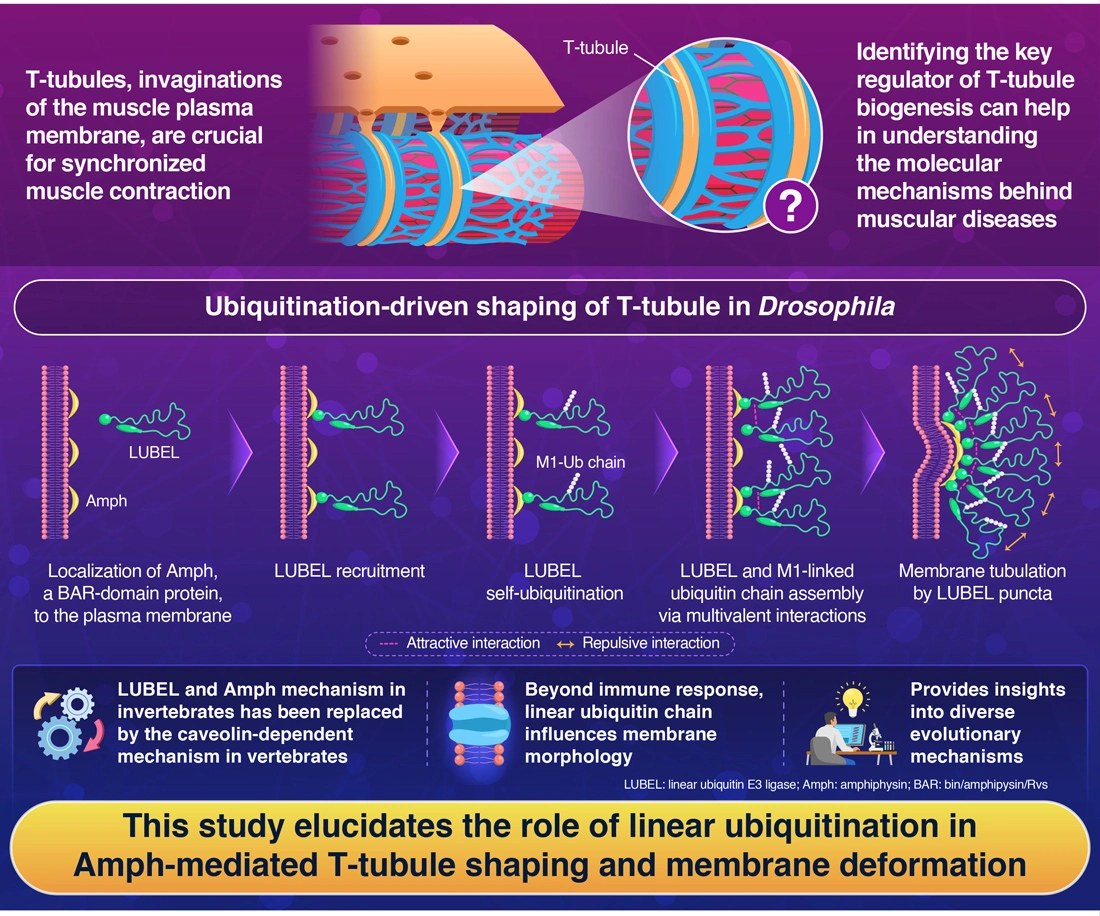
Transverse tubules (T-tubules), the tubular invaginations of the muscle plasma membrane, facilitate synchronized
muscle contraction by transmitting the electrical signals. Despite their significance in muscle physiology, the
mechanisms governing T-tubule formation remain elusive.
Bin/Amphiphysin/Rvs (BAR)-domain proteins are a group of proteins that regulate cellular membrane dynamics by
sensing and bending membrane curvature. They are banana-shaped dimers that bind to the concave surface of curved
membranes. Amphiphysin (Amph) is one such BAR-domain protein associated with T-tubule formation. The N-terminal
BAR-domain of this protein binds to the membrane, while the role of the C-terminal domain in T-tubule shaping had
remained unclear.
A team of researchers led by Associate Professor Naonobu Fujita and Specially Appointed Assistant Professor Kohei
Kawaguchi at the Cell Biology Center, Institute of Science Tokyo (Science Tokyo), Japan, in collaboration with
Professor Hidetaka Kosako at Tokushima University, Japan, and Professor Fumiyo Ikeda at Osaka University, Japan,
used a Drosophila model to conduct experiments and elucidate the underlying mechanism of this T-tubule
biogenesis. Their study was published in the journal Science Advances![]() on January 7, 2026.
on January 7, 2026.
“Drosophila is an ideal model for studying T-tubules. Its genetic accessibility enables detailed analysis of
how these structures are formed, while the fact that T-tubules are not essential for fly survival allows us to study
the mechanisms without affecting viability. In addition, the T-tubule structure can be observed through the cuticle
in live animals,” mentions Fujita, while talking about the reason behind selecting the Drosophila model.
The team performed proximity-dependent labeling proteomics and RNAi screening targeting the T-tubules to elucidate
the molecular mechanism of their formation in the Drosophila model. Proximity-dependent labeling proteomics
refers to a technique in which molecules located very close to a protein of interest are chemically labeled,
purified, and identified to map the protein’s local interaction network. In RNAi screening, expression of the target
genes are suppressed via RNA interference to identify gene functions via the observed phenotypic changes.
The team identified linear ubiquitin E3 ligase (LUBEL), an enzyme responsible for synthesizing linear (M1-linked)
ubiquitin chains, as a novel factor essential for T-tubule biogenesis.
The study findings suggested that in addition to LUBEL’s ubiquitin ligase activity, it also interacts with the
C-terminal domain of Amph, leading to the formation of self-assembled structures composed of LUBEL. Amph bound to
plasma membranes interacts with the LUBEL protein, which initiates the self-ubiquitination process of LUBEL. This
leads to a multivalent interaction among LUBEL molecules through its IDR1 and UBA2 domains, creating a positive
feedback loop that generates LUBEL puncta structures. This entire sequence promotes membrane tubulation by inducing
membrane curvature.
The team also found that, while the cooperative function of LUBEL and Amph is highly conserved among invertebrates,
it has been evolutionarily replaced by caveolin-dependent mechanism in vertebrates. This discovery provides
significant new insights into the evolutionary diversification of T-tubule formation mechanisms.
“Previously linear ubiquitin chains were thought to be involved primarily in immune response. But our findings
suggest that it directly contributes to the regulation of biological membrane morphology,” shares Fujita. The study
proposes a new conceptual framework in which ubiquitination-driven assembly formation regulates the
membrane-remodeling activity of BAR-domain proteins.
Reference
| Authors: | Kohei Kawaguchi1, Yutaro Hama2, Harunori
Yoshikawa3, Kohei Nishino3, Kazuki Morimoto4, Tsuyoshi
Nakamura1, Michiko Koizumi1, Yuriko Sakamaki5, Kota Abe6,
Soichiro Kakuta7, Koichiro Ichimura8, Fumiyo Ikeda9, Hidetaka
Kosako3, and Naonobu Fujita1,4* *Corresponding author |
|---|---|
| Title: | Linear ubiquitination triggers Amph-mediated T-tubule biogenesis |
| Journal: | Science Advances |
| DOI: | 10.1126/sciadv.ady4934 |
| Affiliations: | 1Cell Biology Center, Institute of Science Tokyo,
Japan 2Institute for Genetic Medicine, Hokkaido University, Japan 3Division of Cell Signaling, Tokushima University, Japan 4Graduate School of Life Science and Technology, Institute of Science Tokyo, Japan 5Ochanomizu Research Facility (ORF), Bioscience Center, Research Infrastructure Management Center, Institute of Science Tokyo, Japan 6Department of Homeostatic Regulation, The University of Osaka, Japan 7Laboratory of Morphology and Image Analysis, Juntendo University Graduate School of Medicine, Tokyo, Japan 8Department of Anatomy and Life Structure, Juntendo University Graduate School of Medicine, Japan 9Graduate School of Frontier Biosciences, The University of Osaka, Japan |
Related articles
- Naonobu Fujita | Science Tokyo Research Information DB - Science and engineering fields
- Kohei Kawaguchi | Science Tokyo Research Information DB - Science and engineering fields
- Fujita Laboratory
- Cell Biology Center, Institute of Integrated Research
- Institute of Integrated Research
- Department of Life Science and Technology, School of Life Science and Technology
- School of Life Science and Technology
Further information
Associate Professor Naonobu Fujita
Institute of Integrated Research, Institute of Science Tokyo