Life Science and Technology News
Enzyme found to control formation of collagen carriers and inhibit collagen secretion
Researchers at Tokyo Tech have identified an enzyme that controls how much our cells secrete collagen. As collagen imbalance is linked to a range of human diseases, the study provides clues to new therapeutic strategies. Moreover, the findings could facilitate efficient production of collagen for the food, cosmetic and pharmaceutical industries.
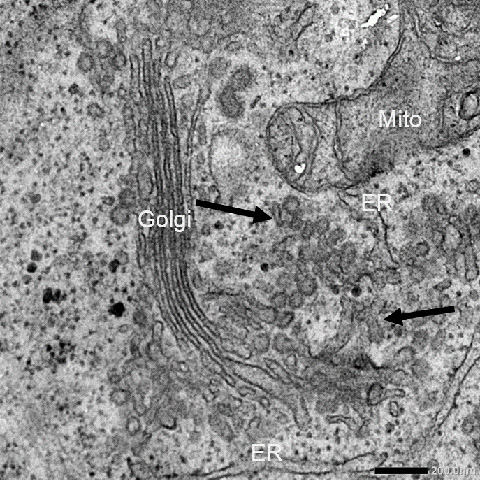
Figure 1. Image of a cell's interior without USP8
Electron microscopy imaging reveals the presence of many interconnected vesicles, which appear to behave like collagen carriers (indicated by arrows).
All of our cells make and release proteins. The proteins are packaged as "cargo" in tiny, bubble-like vesicles before being transported outside the cell. This process, known as secretion, is vital to healthy growth and development.
Although many studies have shown how these vesicles, called COPII carriers1, handle relatively small-sized cargo, few have focused on the workings of unusually large carriers known to package very large proteins, such as collagen.
Now, a study by researchers including Masayuki Komada, Toshiaki Fukushima and graduate student Kohei Kawaguchi at Tokyo Tech has identified USP8 as a key enzyme involved in controlling the formation of large collagen carriers. They have reported their findings in the journal Biochemical and Biophysical Research Communications.
The team showed that "switching on" USP8 inhibited the formation of large carriers, and thus reduced collagen secretion. Conversely, switching USP8 off promoted collagen transport, which led to increased collagen secretion. (See Figures 1-3.)
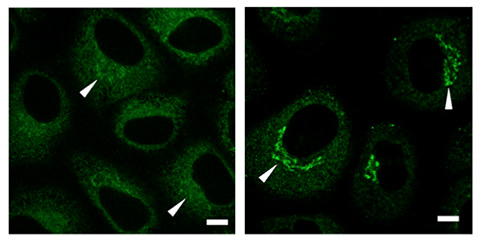
Figure 2. Images of cells with USP8 (left panel) and without USP8 (right panel)
Immunostaining experiments revealed a high concentration of collagen (green signals) in the Golgi region prior to secretion.
The findings have big implications for medicine and biotechnology. Excessive collagen secretion in the human body is known to cause organ fibrosis2, while too little collagen secretion is associated with bone diseases including cranio-lenticulo-sutural dysplasia (CLSD) and Cole-Carpenter syndrome. New treatments for these diseases could be developed through further understanding of USP8's exact mode of action. Such knowledge could also provide new ways of scaling up commercial production of collagen.
The researchers have demonstrated that the enzyme works by deubiquitinating a protein called Sec31A, a component of the COPII vesicle coat required for protein export.
One particular group of proteins called the USP8-STAM1 complex3 appears to be responsible for deubiquitinating Sec31A, as illustrated in Figure 3.
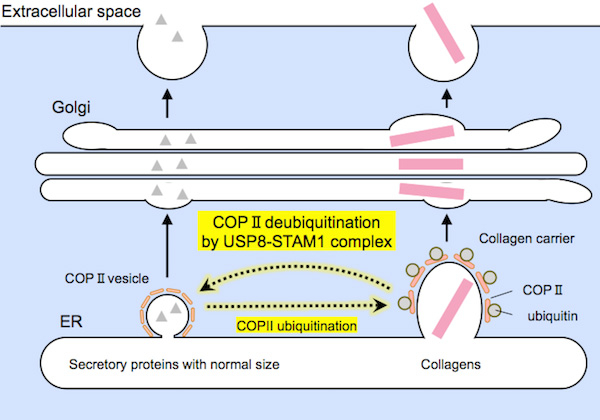
Figure 3. Representation of protein secretion
Relatively small-sized proteins (on the left) and large collagens (on the right) are encapsulated by COPII carriers of suitable sizes prior to secretion. The USP8-STAM1 complex inhibits the formation of large collagen carriers.
The study builds on many years of research that have illuminated the versatility of USP8.
"We had previously reported that USP8 regulates pituitary hormone secretion4," says Fukushima, referring to a paper published in Nature Genetics ![]() in 2015. "In the process of that study, we accidentally found that the USP8-STAM1 complex binds to Sec31."
in 2015. "In the process of that study, we accidentally found that the USP8-STAM1 complex binds to Sec31."
It was this "accidental" finding, combined with promising results from other groups in the US, that led the team to examine the role of USP8 in the formation of COPII carriers.
In research tracing back more than a decade, Komada and others have clarified the conventional role of USP8 in the regulation of endocytosis5. "It's very interesting that the same USP8-STAM1 complex has now been shown to play an important role both in the regulation of endocytosis and in secretion," Fukushima says.
The present study therefore reveals a "new face" of the USP8 enzyme, and Fukushima hints that there may be more surprises to come. USP8 belongs to a family of around 90 known deubiquitinating enzymes, which continue to be a hot topic in cellular biology.
Technical terms
Bubble-like vesicles that wrap up proteins inside cells. They typically have a diameter of 60-70 nanometers (nm), but larger ones are capable of transporting macromolecules such as collagen, which can have a length of 300-400 nm or more.
The development of excessive fibrous tissues, which can lead to organ malfunction.
A group of proteins consisting of the enzyme USP8 and a signal transducing adaptor molecule, STAM1. The latter possesses substrate recognition domains, which enable it to recognize ubiquitinated proteins. These proteins can then be deubiquitinated by USP8.
Referring to hormones secreted by the pituitary gland, at the base of the brain. The USP8 gene is often mutated to generate a hyperactive form of USP8, which can cause excessive secretion of a hormone called ACTH from the pituitary. This excessive secretion is one of the causes of a condition called Cushing's disease.
The transportation of molecules into a cell via its membrane.
Reference
Authors : |
Kohei Kawaguchia, Akinori Endob, Toshiaki Fukushimaa, b, Yuka Madokab, Toshiaki Tanakaa, Masayuki Komadaa, b |
|---|---|
Title of original paper : |
Ubiquitin-specific protease 8 deubiquitinates Sec31A and decreases large COPII carriers and collagen IV secretion |
Journal : |
Biochemical and Biophysical Research Communications 499 (2018) 635-641 |
DOI : |
|
Affiliations : |
a School of Life Science and Technology, Tokyo Institute of Technology b Cell Biology Center, Institute of Innovative Research, Tokyo Institute of Technology |
- Goodbye 'stress granules': Study expands possibilities for treating neurological diseases | Tokyo Tech News
- Choosing the right substrate for the right function: role of ubiquitin-interacting motifs on ubiquitin-specific proteases | Tokyo Tech News
- A new tumor suppressor gene for breast cancer in mice | Tokyo Tech News
- Genetics research demystifies fatal glandular disease | Tokyo Tech News
- Komada Lab
- Researcher Profile | Tokyo Tech STAR Search - Toshiaki Fukushima
- Researcher Profile | Tokyo Tech STAR Search - Masayuki Komada
- Cell Biology Center, Institute of Innovative Research, Tokyo Institute of Technology
- Institute of Innovation research (IIR)
- Latest Research News
Further Information
Assistant Professor Toshiaki Fukushima
Institute of Innovative Research,
Tokyo Institute of Technology
Email tofu@bio.titech.ac.jp
Tel +81-45-924-5702