Life Science and Technology News
A Breakthrough Tool for Detecting Problems During Protein Synthesis
Researchers engineered firefly luciferase-based reporter that can shed light on elusive cellular mechanisms and pathological conditions
A newly developed luciferase-based reporter can detect problems in protein translocation and disulfide bond formation in the endoplasmic reticulum (ER), as reported by researchers at Science Tokyo. Inspired by natural mechanisms found in bacteria, this reporter offers a simple and robust tool for studying ER-related protein synthesis processes, with potential applications in understanding diseases and developing new treatments.
Highly Sensitive Luciferase Reporters Detect Protein Biogenesis Abnormalities
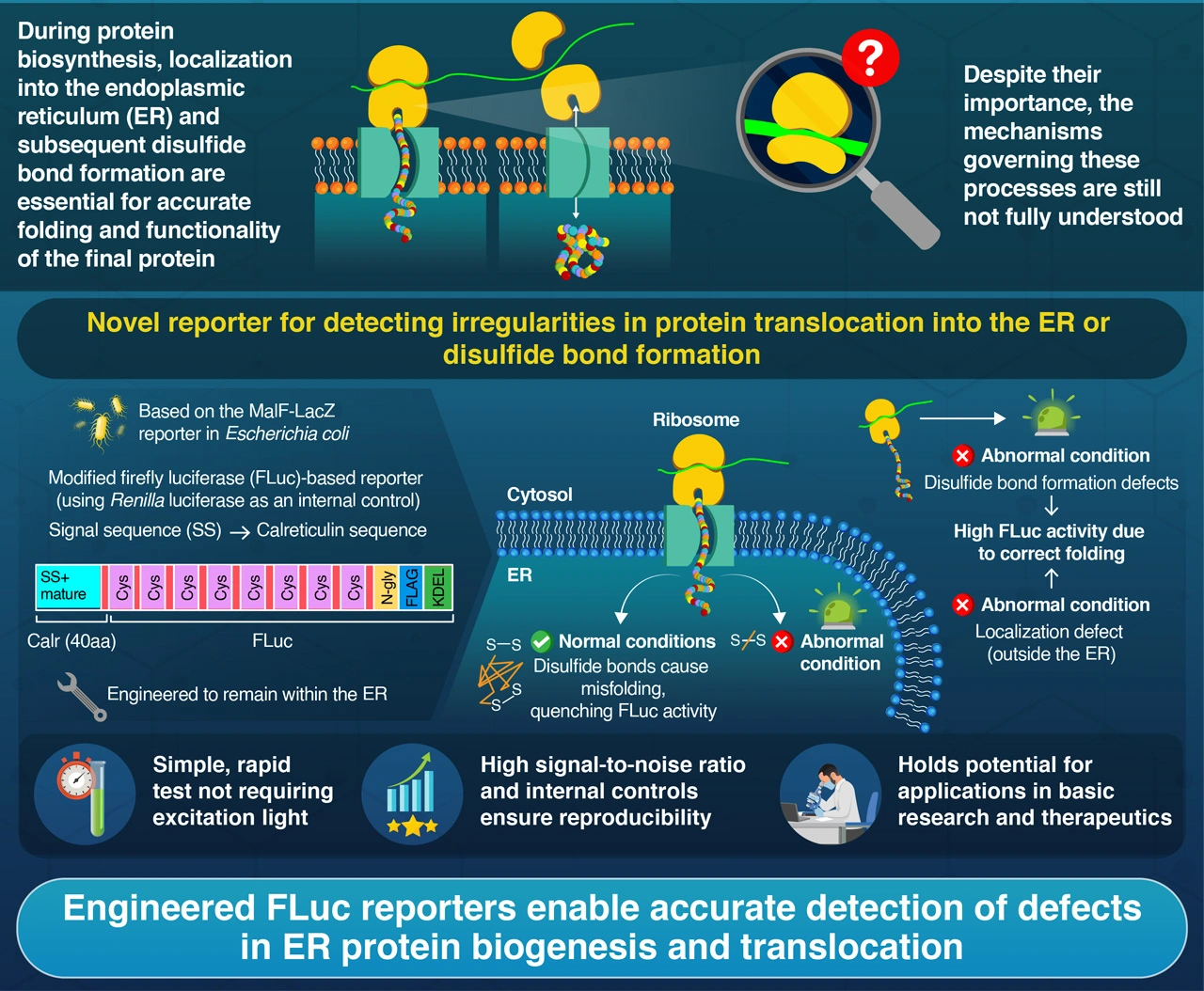
Development of luciferase-based highly sensitive reporters that detect ER-associated protein biogenesis abnormalities![]()
Kadokura et al. (2024) | iScience | 10.1016/j.isci.2024.111189
In eukaryotic cells—found in animals, plants, and fungi—protein synthesis involves more than the simple assembly of amino acids in ribosomes. Nearly one-third of all human proteins must be transported to the endoplasmic reticulum (ER) during or shortly after their synthesis. In the ER, these proteins undergo crucial folding and modifications, including the formation of disulfide (S–S) bonds, which are vital for their structure and function.
Disruptions in protein translocation to the ER or disulfide bond formation underlie several diseases, and understanding the mechanisms that govern these processes is essential in biology and medical science. Unfortunately, the tools available to study them are either quite limited in scope or require exceptionally expensive equipment and carefully repeated measurements.
In an effort to overcome these challenges, a research team including Specially-Appointed Associate Professor Hiroshi Kadokura and Professor Hideki Taguchi from Institute of Science Tokyo, Japan, developed an innovative ‘reporter’ molecule that can detect ER-related problems during protein synthesis. Their findings were published in the journal iScience![]() .
.
While designing this reporter, the researchers took a page from a fusion protein called MalF-LacZ, derived from Escherichia coli bacteria. In these microorganisms, the MalF part of the protein helps with translocating LacZ from the cytoplasm to the cell envelope. Once transported there, the LacZ enzyme undergoes oxidation through disulfide bond formation, thus deactivating it. Therefore, problems in either transportation or disulfide bond formation would result in an abnormally activated LacZ enzyme.
Inspired by these elegant natural mechanisms, the research team developed a reporter molecule based on firefly luciferase (FLuc) that operates in a similar manner. Luciferase is a bioluminescence producing enzyme of firefly that produces light when it catalyzes the oxidation of D-luciferin in the presence of oxygen, adenosine triphosphate (ATP), and magnesium ions (Mg2+). More specifically, they engineered a FLuc variant that is rendered inactive by disulfide bond formation in the ER, but remains active in the cytosol or if disulfide bonds do not form. They ‘targeted’ this compound to the ER by introducing specific modifications, and made it more prone to misfolding (and deactivating) within the ER by strategically replacing amino acids in the FLuc sequence with Cysteine.
Using this reporter, the researchers could easily detect problems in protein translocation to the ER, as well as problems in disulfide bond formation. A bioluminescence producing enzyme of a different type can serve as an internal control and ensures precise measurement. Furthermore, the reporter protein is equipped with a motif that undergoes a modification (glycosylation) only when the protein is translocated into the ER. Thus, they could also determine which of the two possibilities were the underlying cause for the activation of the FLuc reporter.
To showcase the power of this method, the team ran experiments in cells where the redox environment of the ER was chemically altered, disrupting disulfide bond formation. Additionally, they showed that the proposed reporter can detect defects in protein translocation induced by a potential anti-HIV drug, signaling the successful inhibition of the virus. "Given that luciferase-based assays are well-suited for high-throughput platforms, we suggest that this approach will facilitate large-scale screening of small molecules that specifically block the biosynthesis of harmful secretory pathway proteins," highlights Kadokura.
Notably, this novel reporter bears several advantages over other available methods, including its simplicity, robustness against environmental fluctuations, and high reproducibility. "Our reporter system will serve as a valuable tool across various fields related to secretory pathway proteins, extending beyond fundamental studies," says Taguchi, hopeful for the future.
With any luck, these efforts will lead to a better understanding of both life processes and diseases, paving the way to new medical advances and treatments.
- Reference
| Authors: |
Hiroshi Kadokura1,2,3,*, Nanshi Harada1, Satoshi Yamaki1, Naoya Hirai1,3, Ryusuke Tsukuda1, Kota Azuma1, Yuta Amagai1,6, Daisuke Nakamura2, Kota Yanagitani2,4, Hideki Taguchi3, Kenji Kohno2,5, and Kenji Inaba1,6,7
*Corresponding author(s) |
|---|---|
| Title: | Development of luciferase-based highly sensitive reporters that detect ER-associated protein biogenesis abnormalities |
| Journal: | iScience |
| Affiliations: |
1Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Japan 2Institute for Research Initiatives, Nara Institute of Science and Technology, Japan 3Cell Biology Center, Institute of Integrated Research, Institute of Science Tokyo, Japan 4Graduate School of Frontier Biosciences, Osaka University, Japan 5Graduate School of Science, University of Hyogo, Japan 6Medical Institute of Bioregulation, Kyushu University, Fukuoka, Japan 7Core Research for Evolutional Science and Technology (CREST), Japan Agency for Medical Research and Development (AMED), Japan |
| DOI: |
10.1016/j.isci.2024.111189 |
Related articles
- Unveiling Novel Mechanism Underlying the Heat Shock Response in Escherichia coli | Life Science and Technology News
- "Snapshots" of Translation Could Help Us Investigate Cellular Proteins | Life Science and Technology News
- Lost in Translation: How "Risky" Amino Acids Abort Elongation in Protein Synthesis | Life Science and Technology News
- Nascent Polypeptides Stabilize Ribosomes for Uninterrupted Translation, Tokyo Tech Scientists Show | Life Science and Technology News
- How understanding the dynamics of yeast prions can shed light on neurodegenerative diseases | Life Science and Technology News
- Protein intentionally terminates own synthesis by destabilizing synthesis machinery — the ribosome | Life Science and Technology News
- New insights into Protein Synthesis | Formerly Tokyo Tech
- Hideki Taguchi - Chaperones supporting protein life cycles | Formerly Tokyo Tech
- Hiroshi Kadokura|Researcher Finder - Science Tokyo STAR Search
- Hideki Taguchi|Researcher Finder - Science Tokyo STAR Search
- Taguchi Laboratory
- Cell Biology Center, Institute of Integrated Research
- Department of Life Science and Technology, School of Life Science and Technology
- Institute of Integrated Research|Science Tokyo organization|About Science Tokyo
- School of Life Science and Technology|Science Tokyo organization|About Science Tokyo
Further information
Specially-Appointed Associate Professor Hiroshi Kadokura
Institute of Integrated Research, Institute of Science Tokyo