Life Science and Technology News
New Study Sheds Light on the Molecular Mechanisms Underlying Lipid Recycling Within Cells
Our understanding of how cells recycle lipids through autophagy—a form of cellular degradation—has grown significantly, thanks to a recent study by scientists at Tokyo Tech. Using yeast as a model organism, the researchers explored the molecular mechanisms leading to the degradation of the phospholipid bilayers making up the cell membranes. Their findings improve our understanding of cellular degradation processes and related metabolic disorders.
Recycling is just as essential in cells as in our more familiar macroscopic world. Cells continuously generate waste products and accumulate damaged components while performing regular functions. Various recycling mechanisms have evolved to ensure efficient use of these resources and help maintain homeostasis, with autophagy being one of the most well-preserved among countless animal, plant, and fungal lineages.
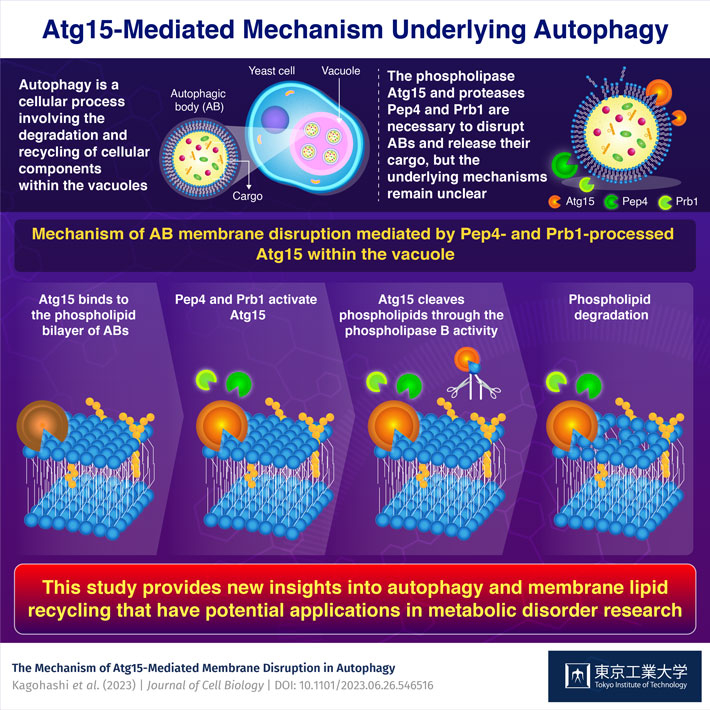
In the main form of autophagy, materials floating in the cell are transported to specialized organelles, such as lysosomes or vacuoles, within small capsule-like structures called autophagosomes. Once these autophagosomes reach inside the lysosomes or vacuoles, they are called autophagic bodies (ABs). To degrade the cargo contained in ABs—a process called autophagy—the proteins within the lysosome or vacuole begin by breaking down the phospholipid bilayers enveloping the ABs. Previous research identified a few key players in this process, namely the proteins Atg15, Pep4, and Prb1. However, the interrelation between these proteins and underlying mechanisms is still unclear.
Recently, a research team from Tokyo Institute of Technology, Japan, has made substantial progress toward solving this puzzle. In a recent study published in the Journal of Cell Biology![]() and led by 2016 Nobel laureate Professor Yoshinori Ohsumi and Assistant Professor Kawamata, they used yeast as a model organism to shed light on some of the intricacies of autophagy. "The relative simplicity of yeast vacuolar enzymes was particularly advantageous for our study as it allowed us to clarify the relationship between protein- and lipid-breaking activity in the vacuole," explains first author Kagohashi.
and led by 2016 Nobel laureate Professor Yoshinori Ohsumi and Assistant Professor Kawamata, they used yeast as a model organism to shed light on some of the intricacies of autophagy. "The relative simplicity of yeast vacuolar enzymes was particularly advantageous for our study as it allowed us to clarify the relationship between protein- and lipid-breaking activity in the vacuole," explains first author Kagohashi.
By applying in vitro assays involving lipid-degradation, the researchers demonstrated that Pep4 and Prb1 transform Atg15 into an 'activated' form. This step is necessary to enable Atg15 to break the phospholipid bilayer of ABs. The team confirmed these findings by testing various Atg15 mutants and yeast strains lacking the genes coding for Pep4 and Prb1. By tagging Atg15 with a probe, they also pinpointed the modifications that Pep4 and Prb1 make to Atg15 within the vacuole.
The team delved deeper into how Atg15 breaks down the phospholipid bilayer through further experiments using isolated ABs. These analyses revealed, for the first time, that Atg15 has phospholipase B activity—this allows Atg15 to cleave phospholipid molecules at two specific locations, thus efficiently disrupting the phospholipid membrane.
In summary, this work deepens our understanding of crucial cellular processes, as Dr. Kawamata remarks: "Characterization of lipid-breaking activity in the vacuole/lysosome is essential to understand how lipids are recycled. This study provides insights into the recycling of membrane lipids and informs work on a range of metabolic disorders." As she notes, autophagy is implicated in many diseases and can also be an attractive drug target for new therapies.
- Reference
| Authors : | Yoko Kagohashi1,2,3, Michiko Sasaki1, Alexander I May1, Tomoko Kawamata1*, and Yoshinori Ohsumi1* |
|---|---|
| Title : | The mechanism of Atg15-mediated membrane disruption in autophagy |
| Journal : | Journal of Cell Biology |
| DOI : | 10.1083/jcb.202306120 |
| Affiliations : |
1 Research Center for Cell Biology, Institute of Innovative Research, Tokyo Institute of Technology 2 School of Life Science and Technology, Tokyo Institute of Technology 3 POLA Chemical Industries Inc. |
| * Corresponding authors' emails: |
- Selective mRNA Degradation via Autophagy: A Novel Role for Autophagy in Gene Regulation | Life Science and Technology News
- Amino Acid Recycling in Cells: Autophagy Helps Cells Adapt to Changing Conditions | Life Science and Technology News
- The role of the Atg2 protein in tethering pre-autophagosomal membranes to the endoplasmic reticulum | Life Science and Technology News
- The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes | Life Science and Technology News
- Yoshinori Ohsumi - Elucidating the mechanism of autophagy | Research Stories | Research
- Alexander Ian May | Researcher Finder - Tokyo Tech STAR Search
- Tomoko Horie | Researcher Finder - Tokyo Tech STAR Search
- Ohsumi Yoshinori | Researcher Finder - Tokyo Tech STAR Search
- Ohsumi Laboratory
- Department of Life Science and Technology, School of Life Science and Technology
- Cell Biology Center
- Organization for Fundamental Research
- Institute of Innovative Research (IIR)
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Learn more about Honorary Professor Yoshinori Ohsumi and his prize-winning research into the mechanisms of autophagy
Special webpage for 2016 Nobel laureate in Physiology or Medicine![]()
Further Information
Professor Yoshinori Ohsumi
Institute of Innovative Research
Tokyo Institute of Technology
Email ohsumi.y.aa@m.titech.ac.jp
Assistant Professor Tomoko Kawamata
Institute of Innovative Research
Tokyo Institute of Technology