Life Science and Technology News
The role of the Atg2 protein in tethering pre-autophagosomal membranes to the endoplasmic reticulum
Postdoctoral Researcher Tetsuya Kotani, Associate Professor Hitoshi Nakatogawa, Honorary Professor Yoshinori Ohsumi and colleagues at Tokyo Institute of Technology have analyzed the Atg protein1 Atg2, whose function had been completely unknown, and have discovered that Atg2 tethers the pre-autophagosomal membrane to the endoplasmic reticulum during autophagosome formation.
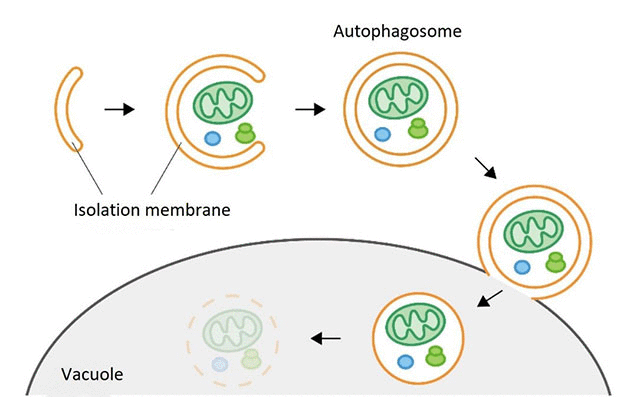
Figure 1. Autophagy process
Autophagy is the mechanism which degrades cellular components such as proteins, ribonucleic acids (RNA) and organelles, and is highly conserved from yeast to humans. When autophagy is induced by stresses such as starvation of nutrients, a flattened membrane vesicle called an isolation membrane appears and expands spherically to engulf materials to be degraded, closing to form a double-membrane vesicle called the autophagosome (Figure 1).
The completed autophagosome then fuses with a lysosome or a vacuole2 and the materials inside the autophagosome are degraded by enzymes inside the lysosome or vacuole. Many Atg proteins playing roles in autophagosome formation have been identified, however, the specific roles of individual Atg proteins in membrane formation are still poorly understood, and the details of the mechanism of autophagosome formation have still not been elucidated.
Overview of Research Achievement
The research group analyzed the function of Atg2, one of the Atg proteins playing a role in autophagosome formation in the budding yeast Saccharomyces cerevisiae. Atg2 is a large protein made of 1,592 amino acids, however, any functional domains are not predicted based on the amino acid sequence. It had been known that Atg2 forms a complex with Atg18, a protein which binds to phosphatidylinositol 3-phosphate (PI3P)3, and that this was the last among Atg proteins to localize at the site of autophagosome formation. However, its specific function had remained unknown.
The comparison of Atg2's amino acid sequence among different organisms revealed that N- and C-terminal regions4 have been highly conserved. The group then prepared many Atg2 variants, focusing on these two regions, and evaluated autophagy activity. As a result, they found that regions which are vital to the function of Atg2 exist in N- and C-terminal regions.
Further, by in vitro experiments using Atg2 purified from yeast cells and liposomes (artifical membrane vesicles), the group demonstrated that both the N- and C-terminal regions of Atg2 have the ability to bind to a membrane, and that Atg2 tethers the two membrane structures together by means of these two membrane-binding regions. They also revealed that the C-terminal region is required for Atg18 to bind to membranes containing PI3P, and that it is required for the Atg2-Atg18 complex to localize to the site of autophagosome formation.
Additionally, they demonstrated that the N-terminal region, in contrast, plays a vital role after the Atg2-Atg18 complex has localized to the site of autophagosome formation, and the possibility that it is involved in the association of Atg2 with the endoplasmic reticulum. In view of the above, the group proposes the model that Atg2 tethers pre-autophagosomal membranes (autophagosome precursors) to the endoplasmic reticulum, initiates autophagosome formation, and mediates membrane expansion (Figure 2).
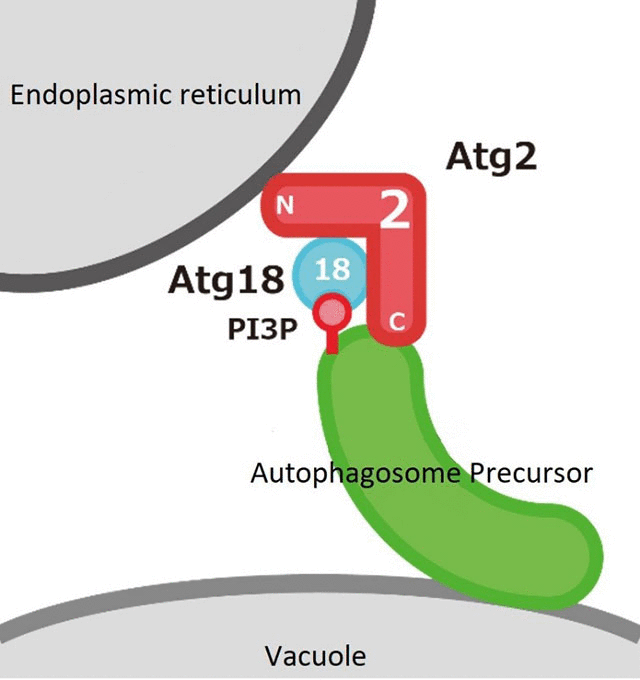
Figure 2. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum
Future Development
Although the formation of the autophagosome with its double-membrane structure is the most unique feature of autophagy, there remain many questions about this process, such as where the membrane comes from and how it is supplied. Previous reports had suggested that the endoplasmic reticulum supplies the membrane for autophagosome formation. Consistent with this model, this research demonstrated the possibility that Atg2 tethers pre-autophagosomal membrane and the endoplasmic reticulum.
However, the mechanism of membrane expansion, such as how lipids are transported from the endoplasmic reticulum to pre-autophagosomal membrane, remains unknown. Analyzing in detail where on the endoplasmic reticulum Atg2 binds and what happens after tethering between pre-autophagosomal membranes and the endoplasmic reticulum is anticipated to lead to unraveling the mechanism of the membrane expansion.
Autophagy is reported to be related to various diseases such as neurodegeneration and cancer. Understanding the mechanism of autophagosome formation is anticipated to provide fundamental information to discovering drugs to treat such autophagy-related diseases.
1 Atg protein: Autophagy-related protein. Over 40 Atg proteins have been reported so far in budding yeast, among which nineteen are involved in autophagosome formation. Most of these were discovered by the group of Professor Yoshinori Ohsumi, the winner of the Nobel Prize in Physiology or Medicine 2016.
2 Lysosome, vacuole: Organelles in the cytoplasm which contain a variety of hydrolases which degrade substances. Lysosomes are present in animal cells, and vacuoles serve this function in plant and yeast cells.
3 Phosphatidylinositol 3-phosphate: A type of phospholipid that has a phosphate group at the hydroxyl group at position 3 of the inositol ring of phosphatidylinositol (PI). During autophagosome formation, a complex of phosphatidylinositol 3-kinase phosphorylates PI to form PI3P.
4 N- and C-terminal regions: Proteins are polymers made of amino acids. When the carboxyl group of one amino acid forms a peptide bond with the amino group of another amino acid, and this is repeated, a linear polymer (protein) is formed. The protein terminus which with the amino group is called the N terminus, and the terminus with the carboxyl group is called the C terminus.
Reference
| Authors : | Tetsuya Kotani1, Hiromi Kirisako1, Michiko Koizumi2, Yoshinori Ohsumi2, Hitoshi Nakatogawa1,* |
|---|---|
| Title of original paper : | The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation |
| Journal : | Proceedings of the National Academy of Sciences of the United States of America |
| DOI : | 10.1073/pnas.1806727115 |
| Affiliations : |
1School of Life Science and Technology, Tokyo Institute of Technology 2Institute of Innovative Research, Tokyo Institute of Technology |
- Tokyo Tech launches new edX MOOC on Autophagy | Tokyo Tech News
- New degradation proteins show route to cell survival | Tokyo Tech News
- Yoshinori Ohsumi - Elucidating the mechanism of autophagy | Tokyo Tech News
- Labs spotlight #7 - Nakatogawa Laboratory - | Life Science and Technology News
- NAKATOGAWA Lab (japanese)
- Ohsumi Lab
- Researcher Profile | Tokyo Tech STAR Search - Hitoshi Nakatogawa
- Researcher Profile | Tokyo Tech STAR Search - Yoshinori Osumi
- School of Life Science and Technology, Department of Life Science and Technology
- Institute of Innovative Research (IIR)
- Cell Biology Center Institute of Innovative Research Tokyo Institute of Technology
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Further Information
Postdoctoral Researcher Tetsuya Kotani,
School of Life Science and Technology
Tokyo Institute of Technology
Email kotani.t.ab@m.titech.ac.jp
Associate Professor Hitoshi Nakatogawa
School of Life Science and Technology
Tokyo Institute of Technology
Email hnakatogawa@bio.titech.ac.jp
Tel +81-45-924-5735