Life Science and Technology News
The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes
Researchers revealed that Atg13 links autophagy initiation factors to each other using a string-like conformation, thereby promoting the association of diverse elements of the autophagy initiation machinery, initiating autophagosome formation through the recruitment of Atg9 vesicles and phosphorylation of various Atg factors.
In order to live, it is necessary for creatures not only to synthesize essential components, but also degrade harmful or superfluous components. Autophagy, an intracellular degradation system conserved among eukaryotes from yeast to humans, contributes to cell homeostasis via isolating and degrading various unnecessary components within cells. Since autophagy dysfunction is linked to severe diseases such as neurodegeneration and cancer, the artificial control of autophagy promises to facilitate the development of therapeutic and preventive treatment for these and severe diseases.
In budding yeast, the Atg1 complex, comprising Atg1, Atg13, Atg17, Atg29 and Atg31, mediates the initiation of autophagy (Figure 1). When autophagy is induced by starvation, these five components gather to form a dimer of pentamers. However, the formation of the dimer of pentamers alone is not sufficient for autophagy induction: and dozens of the pentameric complexes need to assemble into a supramolecular complex of autophagy initiation machinery elements. However, the molecular mechanism has been elusive.
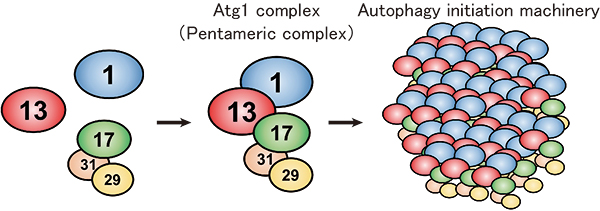
Figure 1.Formation model of the autophagy initiation machinery.
Under nutrient-rich conditions, Atg1, Atg13 and the Atg17-29-31 subcomplex exist without interacting with each other. Upon autophagy induction, under conditions such as starvation, they form a pentameric complex, which further assembles into a giant autophagy initiation machinery complex.
This mystery has been addressed by Honorary Prof. Yoshinori Ohsumi and Dr. Hayashi Yamamoto at Tokyo Institute of Technology, and Drs. Nobuo N. Noda and Yuko Fujioka at Institute of Microbial Chemistry, in collaboration with other researchers. The researchers focused on Atg13 and analysed its structure and function in vitro. The data revealed that Atg13 has an intrinsically disordered, string-like conformation in solution and that Atg13 has two distinct binding regions for Atg17. Detailed analyses of the interaction between Atg13 and Atg17 by X-ray crystallography uncovered that Atg13 links two Atg17 molecules to each other using two binding regions. Analysis of the size of the Atg1 complex revealed that Atg1 complexes are linked to each other by Atg13, which results in the formation of a huge autophagy initiation complex (Figure 2). When point mutations that impair the formation of this giant complex were introduced to Atg13, association of the autophagy initiation machinery was completely blocked. These data suggest that the supramolecular complex resulting from the linkage of Atg1 complexes to each other by Atg13 functions as the autophagy initiation machinery in vivo.
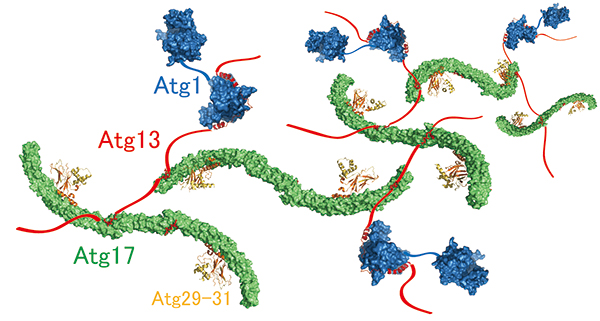
Figure 2.Molecular mechanism of supramolecular autophagy initiaition complex assembly mediated by Atg13.
Atg13 (red) links Atg1 (blue) and two Atg17s (green) to each other, thereby promoting formation of the giant complex.
Observation of "Atg13", a protein highly involved in the autophagy initiation machinery, under high-speed atomic force microscope.
Atg13, which is fused to Maltose-binding protein to enhance visibility, shows swaying string-like conformation. The rotundate portion corresponds to Maltose-binding protein. (Movie captured by Prof. Toshio Ando and Dr. Daisuke Noshiro at Kanazawa University.)
Next, the function of the autophagy initiation machinery was studied in budding yeast, revealing that the phosphorylation activity of Atg1 is markedly enhanced when it is incorporated into the supramolecular autophagy initiation complex. Moreover, it was revealed that the autophagy initiation machinery efficiently recruits Atg9 vesicles, which serve as membrane sources for autophagosome formation, and phosphorylates Atg9. These data collectively suggest that the autophagy initiation machinery not only mediates formation of initial isolation membrane through the recruitment of Atg9 vesicles, but also promotes autophagosome formation via phosphorylating autophagy-related factors including Atg9 (Figure 3).
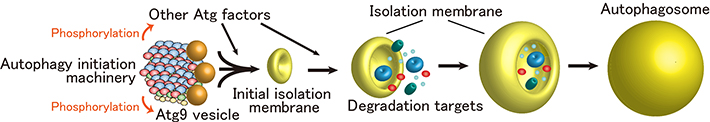
Figure 3.Initiation model of autophagosome formation.
The autophagy initiation machinery mediates formation of the initial isolation membrane through the recruitment of Atg9 vesicles. At the same time, the machinery phosphorylates Atg factors including Atg9, thereby promoting autophagosome formation.
These results are an important step in our quest to fully understand the molecular mechanisms of autophagy initiation and autophagosome formation. A better understanding of the mechanisms of the initial steps of autophagy promises to allow the development of compounds that specifically regulate autophagy, with exciting clinical implications for the treatment of a range of serious conditions.
Reference
| Authors | Yamamoto, H.1, Fujioka, Y.2, Suzuki, S. W.1, Noshiro, D.3, Suzuki, H.2, Kondo-Kakuta, C.1, Kimura, Y.4, Hirano, H.4, Ando, T.3, Noda, N. N.2,*, Ohsumi, Y.1,* *Corresponding authors |
|---|---|
| Title of original paper | The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes |
| Journal | Developmental Cell |
| DOI | 10.1016/j.devcel.2016.06.015 |
| Affiliations | 1 Frontier Research Center, Tokyo Institute of Technology 2 Institute of Microbial Chemistry (BIKAKEN) 3 Department of Physics, College of Science and Engineering, Kanazawa University 4 Advanced Medical Research Center, Yokohama City University |
- Cell Biology Unit
- FACES: Tokyo Tech Researchers, Issue 1 - Yoshinori Ohsumi
- Researcher Profile | Tokyo Tech STAR Search - Yoshinori Ohsumi
- Honorary Professor Yoshinori Ohsumi Receives 2015 Canada Gairdner International Award | Tokyo Tech News
- Institute of Innovative Research
- Institute of Microbial Chemistry
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Further information
Adjunct Professor Yoshinori Ohsumi
Institute of Innovative Research
Email yohsumi@iri.titech.ac.jp
Tel +81-45-924-5113