Chemical Science and Engineering News
A New Design Strategy for Mechanoresponsive Materials with High Thermal Tolerance
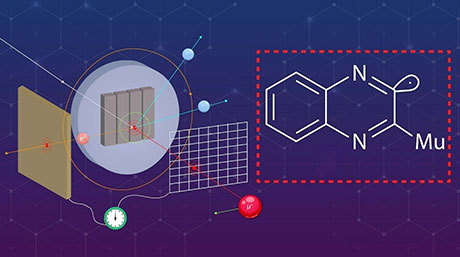
The thermal tolerance of radical-type mechanophores (RMs) that undergo dissociation of the central C–C bond upon mechanical impact increases with the electron-withdrawing ability of the functional group at the para position of the radical skeleton, demonstrate researchers from Tokyo Tech and Sagami Chemical Research Institute. Their study provides a rational design guide for preparing RMs with high thermal tolerance and mechanical responsiveness.
In recent years, scientists have developed a wide range of smart materials known as "mechanoresponsive materials" (MRMs), which exhibit fluorescence, coloring, self-healing, and even self-strengthening in response to mechanical stimuli. One such class of materials is mechanophores—molecules that can undergo small-scale chemical reactions upon exposure to a mechanical stimuli—which are being extensively studied due to their potential application in the fabrication of highly functionalized polymers.
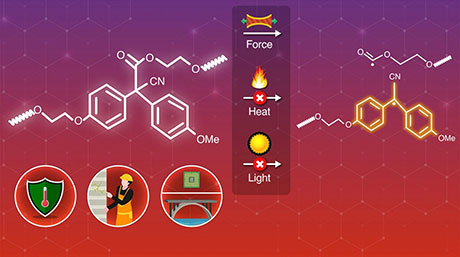
For instance, radical-type mechanophores can undergo mechanical stimuli-induced homolytic scission or breaking of the central carbon–carbon (C–C) bond. Studies have shown that the nature of the C–C bond often decides the heat tolerance, self-healing, chemical reactions for polymeric material preparation and ambient temperature handling of the resulting polymer materials. To improve the reliability of RMs, it is necessary to ensure high stability under thermal stimuli while retaining their mechanical responsiveness. However, there is a lack of rational design strategies for the synthesis of novel RMs.
In a recent breakthrough published in Chemical Science![]() on 3 August 2023, a team of researchers led by Professor Hideyuki Otsuka from Tokyo Institute of Technology (Tokyo Tech) have addressed this problem. They present a new design strategy for developing RMs with high thermal tolerance and good mechanoresponsive ability. "The relationship between thermal and mechanical scission is still unclear, which limits the development of RMs with pre-determined properties. So, we decided to investigate the factors that determine the thermal tolerance behavior of RMs," explains Prof. Otsuka when asked about the motivation behind their study.
on 3 August 2023, a team of researchers led by Professor Hideyuki Otsuka from Tokyo Institute of Technology (Tokyo Tech) have addressed this problem. They present a new design strategy for developing RMs with high thermal tolerance and good mechanoresponsive ability. "The relationship between thermal and mechanical scission is still unclear, which limits the development of RMs with pre-determined properties. So, we decided to investigate the factors that determine the thermal tolerance behavior of RMs," explains Prof. Otsuka when asked about the motivation behind their study.
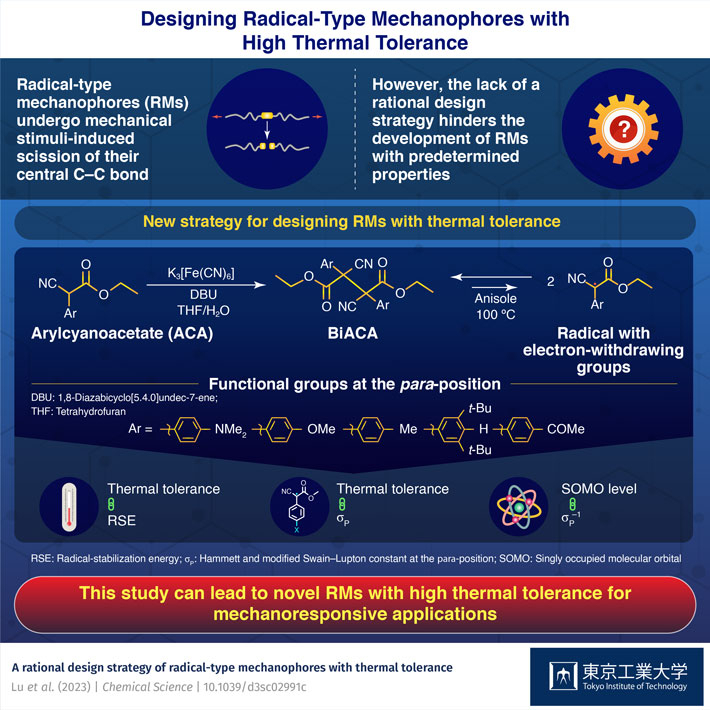
The team selected eight different radical skeletons, each of which was substituted with eight different functional groups. A combined theoretical and experimental analysis showed that for an RM series with the same radical skeleton, the thermal tolerance could be explained by two main factors: radical-stabilization energy, which represents the thermodynamic stability of the radicals during the reactions, and the Hammett and modified Swain–Lupton constants at the para-position (σp), which correspond to the electron-withdrawing or donating ability of the functional groups at the para-position of an aromatic compound.
The researchers hypothesized that the thermal tolerance of RMs improves with the electron-withdrawing ability of the functional groups. They successfully validated their hypothesis experimentally by synthesizing RMs based on bisarylcyanoacetate skeletons. Furthermore, the study revealed a negative correlation between the singly occupied molecular orbital levels and the σp values within an RM series based on a common skeleton.
The systematic design guideline presented in the current study opens up new pathways for the development of novel RMs with pre-determined functionalities, such as high thermal tolerance and protection against peroxidation in ambient conditions. "Apart from their applications for functional materials, RMs can also facilitate the study of micro-to-macroscale mechanical stimuli, strengthened materials based on sacrificial bonds, and subsequent polymer propagation," says Prof. Otsuka. "We believe that our new design strategy will encourage scientists to explore different ways in which RMs can be used for both practical and fundamental research purposes," he concludes.
- Reference
| Authors : | Yi Lu1, Hajime Sugita1,2, Koichiro Mikami2,*, Daisuke Aoki1, and Hideyuki Otsuka1,3,* |
|---|---|
| Title : | A rational design strategy of radical-type mechanophores with thermal tolerance |
| Journal : | Chemical Science |
| DOI : |
10.1039/d3sc02991c |
| Affiliations : |
1 Department of Chemical Science and Engineering, Tokyo Institute of Technology 2 Sagami Chemical Research Institute 3 Living Systems Materialogy (LiSM) Research Group, International Research Frontiers Initiative (IRFI), Tokyo Institute of Technology |
|
* Corresponding authors' emails: r200870208tyoko@gmail.com (K.M.); otsuka@mac.titech.ac.jp (H.O.) |
|
- Studying Polymer Gels Through the Lens of Mechanochemistry and Solvent Swelling | Tokyo Tech News
- Plant from Plastics: Bio-based Polymers Can Be Transformed into Fertilizers | Tokyo Tech News
- Novel Peroxide-based Material Emits Fluorescence in Response to Stress | Tokyo Tech News
- A Show of Force: Novel Polymer that Toughens Up and Changes Color Upon Mechanical Stress | Tokyo Tech News
- Mechanophores: Making Polymer Crystallization Processes Crystal Clear | Tokyo Tech News
- Taking a Shine to Polymers: Fluorescent Molecule Betrays the Breakdown of Polymer Materials | Tokyo Tech News
- Mixing the unmixable —A novel approach for efficiently fusing different polymers | Tokyo Tech News
- Otsuka Laboratory
- Hideyuki Otsuka | Researcher Finder - Tokyo Tech STAR Search
- Chemical Science and Engineering Graduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Chemical Science and Engineering Undergraduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Latest Research News | Tokyo Tech News
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Professor Hideyuki Otsuka
School of Materials and Chemical Technology
Tokyo Institute of Technology
Email otsuka@mac.titech.ac.jp