Chemical Science and Engineering News
Building Molecular Bridges: New Crystal Engineering Strategy to Design Ultrabright Fluorescent Solid Dyes
When it comes to designing ultrabright solid-state fluorescent materials, bridged crystal designs might be the key to enabling monomeric emission and accessing novel crystalline systems, reveals a new study. In the study, a research team from Tokyo Institute of Technology prepared ultrabright fluorescent dyes using di-bridged distyrylbenzenes (DSBs) with flexible alkylene bridges, using a novel crystal engineering study. The findings are sure to have important implications for the field of photofunctional materials.
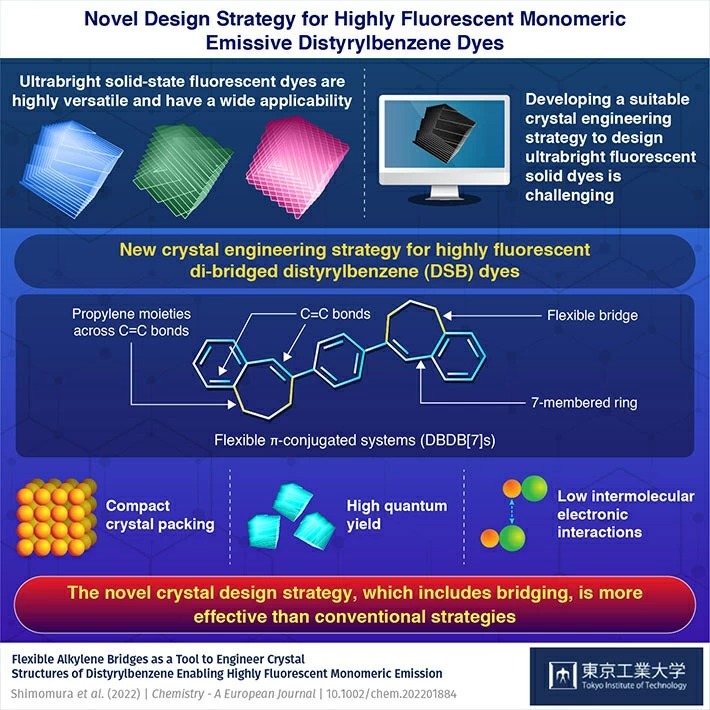
Fluorescent solid organic dyes have an array of applications ranging from functional nanomaterials and organic light-emitting diode (OLED) displays to lasers and bio-imaging. These molecules have excellent versatility, adaptable molecular designs, and excellent processability. Improving the luminescent properties, crystallinities, and emission colors of these solid-state fluorescent dyes is a key area of research in the field, especially for the design of advanced OLEDs. However, developments to this end are limited by three major factors. One, most fluorescent dyes experience concentration quenching (a reduction in fluorescence when the concentration of the fluorescing molecule exceeds a certain level) in the solid state. Two, the tendency of dye molecules to aggregate in the solid state and produce fluorescence of different colors due to the resulting intermolecular electronic interactions. And three, crystal design strategies that can ensure monomeric emission (essentially, emissions of a single wavelength, i.e., color) are underdeveloped.
To address this, a research team, led by Associate Professor Gen-ichi Konishi of Tokyo Institute of Technology, developed a novel crystal design strategy using flexible molecular bridges. The study, published in Chemistry - A European Journal![]() , describes the preparation highly fluorescent monomeric emissive di-bridged distyrylbenzenes (DSBs) with controlled electronic properties and luminescence. "A typical approach to crystal design for fluorescent solid dyes is the steric-hindrance-based strategy, where we manipulate the bulk of a molecule to cause congestion around the reactive atoms and suppress intermolecular interactions. But a frequent disadvantage of this approach is an increased distance between the chromophores (fluorescent molecules). Our design strategy successfully avoids this side effect," explains Associate Prof. Konishi.
, describes the preparation highly fluorescent monomeric emissive di-bridged distyrylbenzenes (DSBs) with controlled electronic properties and luminescence. "A typical approach to crystal design for fluorescent solid dyes is the steric-hindrance-based strategy, where we manipulate the bulk of a molecule to cause congestion around the reactive atoms and suppress intermolecular interactions. But a frequent disadvantage of this approach is an increased distance between the chromophores (fluorescent molecules). Our design strategy successfully avoids this side effect," explains Associate Prof. Konishi.
In this study, the research team prepared a highly dense crystalline structure called DBDB[7]s. DSBs and DBDB[7]s are π-conjugated systems, meaning these organic molecules have alternating single bonds (C-C) and double bonds (C=C) in their structures. The team introduced an organic functional group called propylene as bridge molecules in between the six-membered rings on either side of the double bonds in the DSB structure. This addition gave rise to a new compact crystal structure with suppressed intermolecular interactions and lower distances between the chromophores. "Essentially, the introduction of seven-membered (after bridging) rings to the DSB core created a moderate distortion and steric hindrance in the π-plane of DSB, which allowed us to control the molecular arrangement without increasing the crystal density," says Associate. Prof. Konishi.
The team further investigated the photophysical properties of DBDB[7]s and discovered that small size of the bridge molecules used in this study aided monomeric emission in the solid-state. They also saw that DBDB[7]s was ultrabright with high quantum yield and emitted similar colors in both unaggregated dilute solution and in solid-state.
"The bridged DSB crystal structure described in our study allows access to novel crystalline systems," concludes Associate Prof. Konishi. "Our strategy has far-reaching implications for how we approach the design of photofunctional molecular crystals."
A bridge towards brighter displays indeed!
- Reference
| Authors : | Yoshimichi Shimomura1, Kazunobu Igawa2, Shunsuke Sasaki3, Noritaka Sakakibara4, Raita Goseki5, Gen-ichi Konishi1,6 |
|---|---|
| Title of original paper : | Flexible Alkylene Bridges as a Tool To Engineer Crystal Distyrylbenzene Structures Enabling Highly Fluorescent Monomeric Emission |
| Journal : | Chemistry - A European Journal |
| DOI : | 10.1002/chem.202201884 |
| Affiliations : | 1Department of Chemical Science and Engineering, Tokyo Institute of Technology 2Institute for Materials Chemistry and Engineering, Kyushu University 3Université de Nantes, CNRS, Institut des Matériaux Jean Rouxel 4Department of Chemistry, Tokyo Institute of Technology 5Department of Applied Chemistry, Kogakuin University 6PRESTO "Element Strategy", Japan Science and Technology Agency(JST) |
- New functional dye for realizing a versatile two-photon excitation microscope | Tokyo Tech News
- Academic year 2020 Best Teacher Award recipients celebrated | Tokyo Tech News
- Konishi laboratory
- Gennichi Konishi | Researcher Finder - Tokyo Tech STAR Search
- Chemical Science and Engineering Graduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Chemical Science and Engineering Undergraduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Department of Chemistry, School of Science
- Institute for Materials Chemistry and Engineering, Kyushu University
- Nantes Université
- Kogakuin University
- Latest Research News
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Associate Professor Gen-ichi Konishi
School of Materials and Chemical Technology,
Tokyo Institute of Technology