Life Science and Technology News
Elucidating the mechanism that prevents cell death caused by uncontrolled nuclear autophagy
Researchers examine how macronucleophagy ensures survival in nitrogen-starved yeast
Macronucleophagy helps control micronucleophagy to protect yeast cells during stress. Through extensive experiments with yeast mutants, researchers from Science Tokyo found that disrupting macronucleophagy triggers excessive micronucleophagy, causing cell death. They further identified that this hyperactivation is driven by the accumulation of Nuclear vacuolar junction (Nvj1), a key nuclear membrane protein. These findings shed light on the links between different autophagy pathways under challenging conditions, which are crucial to maintain cell health.
Elucidating the Mechanism Preventing Cell Death Caused by Uncontrolled Nuclear Autophagy
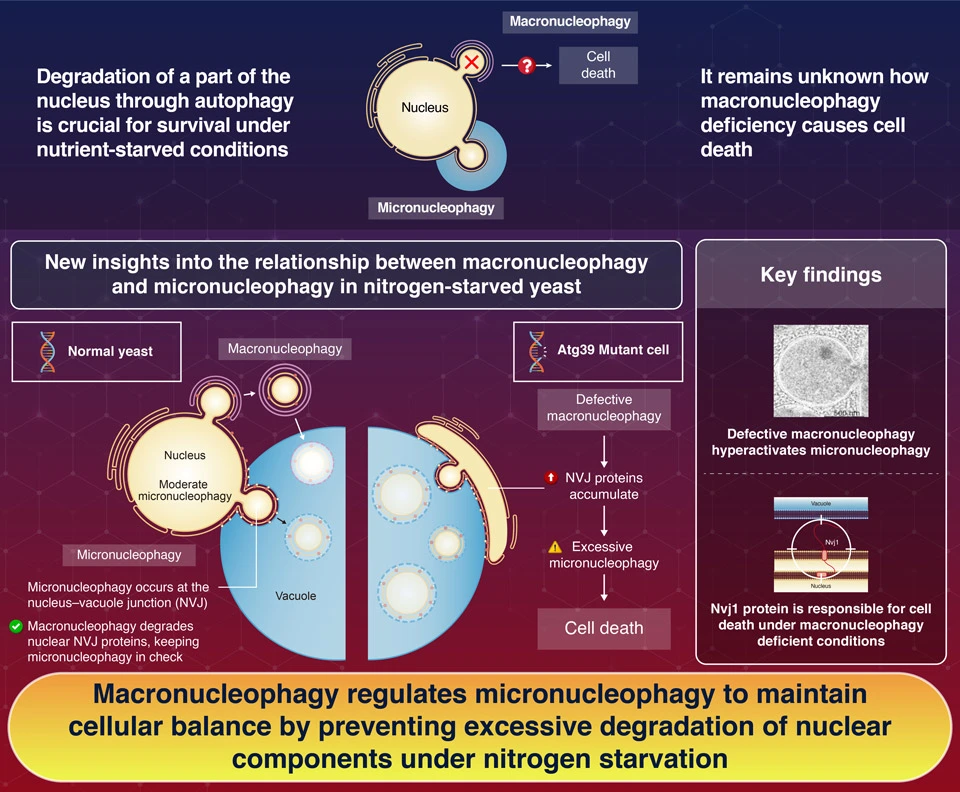
Macronucleophagy maintains cell viability under nitrogen starvation by modulating micronucleophagy![]()
Li et al. (2024) | Nature Communications
Autophagy, the cell’s essential ‘housekeeping’ process, involves degrading and recycling damaged organelles, proteins, and other components to prevent clutter. This vital mechanism, found in all life forms from single-celled organisms to plants and animals, is key for maintaining cellular homeostasis. Its disruption is linked to many known diseases in humans, such as Alzheimer’s, Parkinson’s, and cancer.
Though understanding autophagy in detail is important from medical and biological perspectives, it is not a one-size-fits-all process. There are several forms of autophagy that differ in how the components to be degraded are transported to the lysosomes or vacuoles—the organelles that serve as the cell’s waste disposal and recycling centers. Autophagy targets a range of intracellular components including a part of the nucleus that stores important chromosomes. However, the physiological significance of autophagic degradation of the nucleus remains unknown.
Against this backdrop, a research team from Japan recently focused on an autophagy pathway that degrades a part of the nucleus (macronucleophagy) in Saccharomyces cerevisiae, a species of yeast widely used as a model organism in cellular research. In their latest paper, published in Nature Communications![]() , the scientists sought to shed light on how macronucleophagy ensures the survival of nitrogen-starved S. cerevisiae and revealed that uncontrolled micronucleophagy due to the lack of normal macronucleophagy causes cell death. This work was led by PhD student Ziyang Li from Institute of Science Tokyo.
, the scientists sought to shed light on how macronucleophagy ensures the survival of nitrogen-starved S. cerevisiae and revealed that uncontrolled micronucleophagy due to the lack of normal macronucleophagy causes cell death. This work was led by PhD student Ziyang Li from Institute of Science Tokyo.
To clarify, macronucleophagy is an autophagy pathway in which a part of the nucleus bulges outwards, is encapsulated by a vesicle called ‘autophagosome’, and is delivered to the vacuole. In contrast, in micronucleophagy, the nucleus’ outer surface directly contacts the vacuole at nuclear-vacuole junctions (NVJs). With the help of specific nuclear and vacuolar membrane proteins, a portion of the nucleus pushes into the vacuole (or ‘invaginates’), splits, and is finally degraded.
In a previous study, the research team found that mutant yeast cells with defective macronucleophagy (atg39Δ mutants) died rapidly when placed under nitrogen starvation. After careful comparisons of wild-type and atg39Δ cells, the researchers revealed that micronucleophagy was abnormally increased in the mutants, and this hyperactivation was a major contributor to cell death.
Through further analysis, the team linked this hyperactivation to a nuclear surface protein called Nvj1, which plays a key role in the tethering of the nucleus and the vacuole at the NVJs. It turns out that problems in macronucleophagy lead to the accumulation of Nvj1 on the nucleus, which in turn greatly enhances micronucleophagy. “Blocking micronucleophagy, almost completely suppressed cell death caused by the absence of macronucleophagy, whereas enhancing micronucleophagy exacerbated cell death under nitrogen starvation,” comments Li. “On the other hand, these results suggest that macronucleophagy modulates micronucleophagy in order to prevent the excess removal of nuclear components, thereby maintaining nuclear and cellular homeostasis.”
Taken together, the findings of this study shed light on how different forms of autophagy are closely related, even to the point where they dictate cell survival under stress. “We revealed that the modulation of micronucleophagy is a critical role for macronucleophagy, providing a physiological and mechanistic linkage between these distinct autophagy pathways,” concludes Li. Further studies on this complex topic will help us understand how cells take care of themselves, which could have important implications in medicine and biotechnology.
- Reference
| Authors: | Ziyang Li1,2, Keisuke Mochida1,2, Hitoshi Nakatogawa1,2* |
|---|---|
| Title: | Macronucleophagy maintains cell viability under nitrogen starvation by modulating micronucleophagy |
| Journal: | Nature Communications |
| DOI: |
10.1038/s41467-024-55045-9 |
| Affiliations: |
1 Cell Biology Center, Institute of Integrated Research, Institute of Science Tokyo, Japan 2 School of Life Science and Technology, Institute of Science Tokyo, Japan |
Related articles
- Cellular Cleanup! Atg40 Folds the Endoplasmic Reticulum to Facilitate Its Autophagy | Life Science and Technology News
- The role of the Atg2 protein in tethering pre-autophagosomal membranes to the endoplasmic reticulum | Life Science and Technology News
- New degradation proteins show route to cell survival | Formerly Tokyo Tech
- Hitoshi Nakatogawa | Researcher Finder - Science Tokyo STAR
- Nakatogawa Laboratory (Japanese)
- Cell Biology Center, Institute of Integrated Research
- Institute of Integrated Research | Science Tokyo organization | About Science Tokyo
- Department of Life Science and Technology, School of Life Science and Technology
Further Information
Professor Hitoshi Nakatogawa
Institute of Integrated Research, Institute of Science Tokyo