Life Science and Technology News
Cellular Cleanup! Atg40 Folds the Endoplasmic Reticulum to Facilitate Its Autophagy
Scientists at Tokyo Institute of Technology (Tokyo Tech) and Institute of Microbial Chemistry investigated "ER-phagy," the degradation mechanism of the endoplasmic reticulum (ER), an important organelle with multiple biologically necessary functions like the synthesis of proteins and lipids. Degradation is critical for maintaining ER functions. Scientists found that the "Atg40" protein not only marks ER parts to be degraded by autophagy, but also folds them for efficient degradation, contributing to our understanding of a critical process in cellular maintenance.
The endoplasmic reticulum (ER) is an important part of eukaryotic cells (the type of cells that make up every living thing other than bacteria or viruses, including humans). They are a mass of tubes connected to the nucleus of the cell; the production of both proteins and lipids occur in the networks of the ER. For this organelle to properly function, cells routinely degrade portions of the ER so that it can be renewed. This process is called ER autophagy, or ER-phagy, where a structure called an "isolation membrane" expands and closes up to form an "autophagosome." The closure isolates various cellular materials including the ER within the autophagosome, which then transports the waste away for degradation.
While this process can be random, scientists have uncovered "autophagy receptors" that bind specifically to certain targets and interact with a group of proteins called Atg8, located on the isolation membrane. This interaction allows cells to target specific parts for degradation. In yeast, an organism commonly used for biological research, scientists have identified the protein Atg40 as an ER-phagy receptor, and also found that parts of its structure share similarities to a group of proteins called DP1/Yop1 (reticulon-like proteins), which "curves" the ER membranes into shape and maintains their tubular structures.
"Our previous work reveals that Atg40 is important for ER-phagy, but we actually know very little about how the process works," explained Dr. Hitoshi Nakatogawa of the Tokyo Tech, who led a team of scientists in research that investigated the mechanisms of Atg40 involvement in ER-phagy. "Because degradation of ER is so important for proper cellular function, gaining a better understanding of ER-phagy will improve basic biological knowledge."
Their experiments with yeast, the findings of which are published in Nature Communications, showed that Atg40 is important for "curving and folding" the ER membrane, and therefore has a similar function to DP1/Yop1, explaining their structural similarities. Atg40 is also necessary for ER-phagy, specifically being involved in breaking up the ER membrane so that it can be imbibed by autophagosomes. Researchers demonstrated that during this folding and fragmentation of the ER, Atg40 forms a protein assembly (cluster of proteins) by interacting with Atg8 located specifically at points of contact between the ER and the isolation membrane (as shown in Figure 1). In other words, Atg40 does not randomly or always remodel ER structure; it does so only for the ER parts that will be degraded.
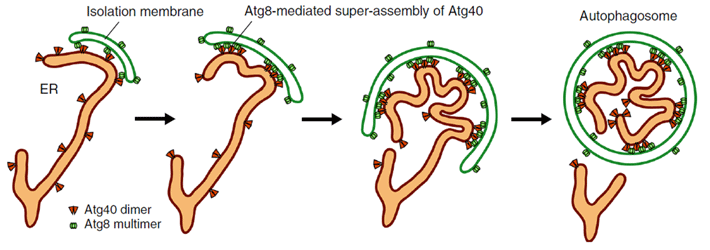
Figure 1. Model for Atg40 action during ER-phagy
Atg40 binds to Atg8 on the isolation membrane. This process changes ER membrane shape so that it can be packed into the isolation membrane, forming an autophagosome that then degrades the ER parts.
Regarding the significance of these results, Dr. Nakatogawa commented: "What I find particular exciting is the insight we gained on a crucial part of how cells work, how they deal with waste or get rid of abnormal cell parts. Our work doesn’t just have implications for ER-phagy though, it can also potentially tell us something about how other organelles, like the nucleus or mitochondria, are degraded."
Besides just being valuable basic research, these findings also have significant practical applications. Knowing the mechanisms of organelle degradation might help the development of drugs that target this process if it breaks down. This presents potential attractive solutions for diseases involving the malfunction of ER such as sensory neuropathy.
- Reference
| Authors : | Keisuke Mochida1#, Akinori Yamasaki2,3#, Kazuaki Matoba2, Hiromi Kirisako1, Nobuo N. Noda2*, Hitoshi Nakatogawa1* |
|---|---|
| Title of original paper : | Super-assembly of ER-phagy receptor Atg40 induces local ER remodeling at contacts with forming autophagosomal membranes |
| Journal : | Nature Communications |
| DOI : | |
| Affiliations : |
1School of Life Science and Technology, Tokyo Institute of Technology 2Institute of Microbial Chemistry (BIKAKEN) 3Present address: Cell Biology Center, Institute of Innovative Research, Tokyo Institute of Technology #Co-first authors |
| * Corresponding author's email: |
nn@bikaken.or.jp; ; (NN); hnakatogawa@bio.titech.ac.jp (HN) |
- The role of the Atg2 protein in tethering pre-autophagosomal membranes to the endoplasmic reticulum | Life Science and Technology News
- Tokyo Tech launches new edX MOOC on Autophagy | Life Science and Technology News
- New degradation proteins show route to cell survival | Tokyo Tech News
- Professor Ohsumi attends Nobel Prize Award Ceremony and Banquet | Tokyo Tech News
- 【Labs spotlight】 Nakatogawa Laboratory - | Life Science and Technology News
- Nakatogawa Lab. (Japanese)
- Researcher Profile | Tokyo Tech STAR Search - Hitoshi Nakatogawa
- Researcher Profile | Tokyo Tech STAR Search - Akinori Yamasaki
- Department of Life Science and Technology, School of Life Science and Technology
- Institute of Microbial Chemistry
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Further Information
Associate Professor Hitoshi Nakatogawa
School of Life Science and Technology, Tokyo Institute of Technology
E-mail : hnakatogawa@bio.titech.ac.jp
Tel +81-45-924-5735