Life Science and Technology News
Researchers Uncover How Photosynthetic Organisms Regulate and Synthesize ATP
The redox regulation mechanism responsible for efficient production of ATP under varying light conditions in photosynthetic organisms has now been unveiled by Tokyo Tech researchers. They investigated the enzyme responsible for this mechanism and uncovered how the amino acid sequences present in the enzyme regulate ATP production. Their findings provide valuable insights into the process of photosynthesis and the ability to adapt to changing metabolic conditions.
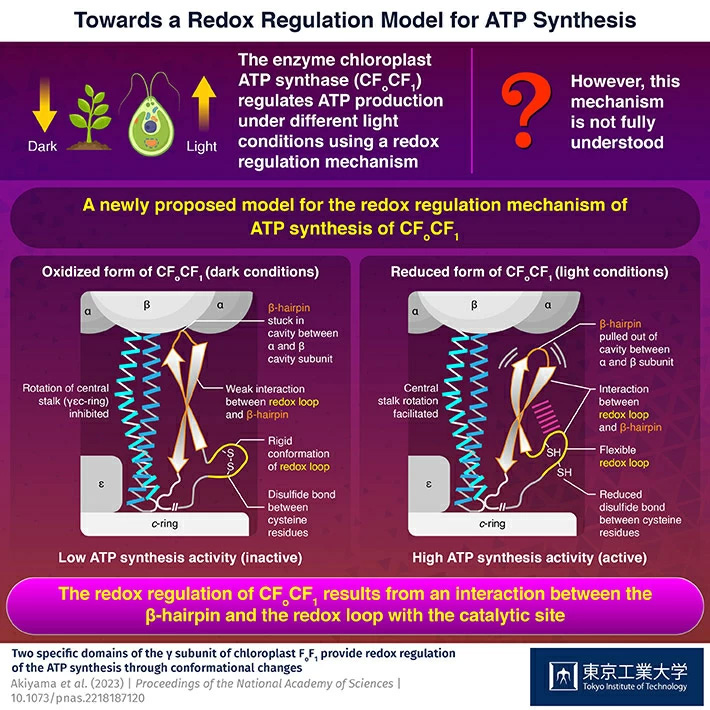
ATP, the compound essential for the functioning of photosynthetic organisms such as plants and algae, is produced by an enzyme called "chloroplast ATP synthase" (CFoCF1). To control ATP production under varying light conditions, the enzyme uses a redox regulatory mechanism that modifies the ATP synthesis activity in response to changes in the redox state of cysteine (Cys) residues, which exist as dithiols under reducing (light) conditions, but forms a disulfide bond under oxidizing (dark) conditions. However, this mechanism has not been fully understood so far.
Now, in a study published in the Proceedings of the National Academy of Sciences, a team of researchers from Japan, led by Prof. Toru Hisabori from Tokyo Institute of Technology (Tokyo Tech), has uncovered the role of the amino acid sequences present in CFoCF1, revealing how the enzyme regulates ATP production in photosynthetic organisms.
To understand how the conformation of the amino acids present in CFoCF1 contributes to the redox regulation mechanism, the researchers used the unicellular green alga, Chlamydomonas reinhardtii, to produce the enzyme. "By leveraging the powerful genetics of Chlamydomonas reinhardtii as a model organism for photosynthesis, we conducted a comprehensive biochemical analysis of the CFoCF1 molecule," explains Prof. Hisabori.
With the alga as the host organism, the team introduced plasmids (extrachromosomal DNA molecule that can replicate independently) that encoded the F1 component of the CFoCF1 protein, namely the part of the enzyme containing catalytic sites for ATP synthesis. They additionally introduced mutated versions of the gene to change the amino acid sequences of the protein, specifically targeting the DDE motif (a cluster of negatively charged amino acids), the redox loop, and the β-hairpin domain.
They then purified CFoCF1, generating five different variations of it that included a wild-type strain with no changes to the amino acid sequence and four mutant strains: one with the DDE motif replaced with neutral amino acids, Asn-Asn-Gln, one without the β-hairpin domain, one without the redox loop, and one lacking both the redox loop and the β-hairpin domain.
Upon testing the ATP synthesis activity of these mutants under reducing (mimicking the light conditions) and oxidizing (mimicking the dark conditions) conditions, the researchers found that the wild-type enzyme and the mutant enzyme with changes to the DDE motif functioned normally (showed high activity when reduced and low activity when oxidized). However, the enzyme complexes without the redox loop or the β-hairpin domain did not show the redox-response, indicating that both regions were involved in the redox regulation mechanism.
The researchers suggested that under dark conditions, the disulfide bond between the Cys residues makes the redox loop rigid and weakens the interaction between the redox loop and the β-hairpin. This causes the β-hairpin to remain stuck within a cavity in the protein. However, when the disulfide bond is reduced in presence of light, the redox loop regains its flexibility and pulls the β-hairpin out of the cavity, enabling it to partake in the ATP synthesis activity.
"The redox regulation of ATP synthesis is accomplished by a cooperative interaction between two γ subunit domains of CFoCF1 unique to photosynthetic organisms," says Prof. Hisabori. "We propose that it results from the interaction of the β-hairpin and the redox loop with the catalytic site."
The results are an important step towards understanding the photosynthesis process better, with the potential for significant implications in the fields of agriculture and bioenergy.
- Reference
| Authors : | Kentaro Akiyama1,2, Shin-Ichiro Ozawa3, Yuichiro Takahashi4, Keisuke Yoshida1,2, Toshiharu Suzuki1,5, Kumiko Kondo1, Ken-ichi Wakabayashi1,2,*, and Toru Hisabori1,2,* |
|---|---|
| Title : | Two specific domains of the γ subunit of chloroplast FoF1 provide redox regulation of the ATP synthesis through conformational changes |
| Journal : | Proceedings of the National Academy of Sciences of the United States of America |
| DOI : | 10.1073/pnas.2218187120 |
| Affiliations : | 1 Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology 2 School of Life Science and Technology, Tokyo Institute of Technology 3 Institute of Plant Science and Resources, Okayama University 4 Research Institute for Interdisciplinary Science, Okayama University 5 Molecular Physiology Laboratory, Cluster for Pioneering Research, RIKEN |
| * Corresponding authors' emails: | wakaba@res.titech.ac.jp (Ken-ichi Wakabayashi) ; thisabor@res.titech.ac.jp (Toru Hisabori) |
- Genetically encoded sensor tracks changes in oxygen levels with very high sensitivity | Life Science and Technology News
- How do plants rest photosynthetic activity at night? | Life Science and Technology News
- Linking light to life: new pathways that help plants adapt to changing environments | Life Science and Technology News
- Shedding light on phototactic behavior of green algae | Life Science and Technology News
- Gene regulation boosts ammonia production | Tokyo Tech News
- Visualization of redox states of protein in vivo | Tokyo Tech News
- 【Labs spotlight】 Hisabori and Wakabayashi Laboratory | Life Science and Technology News
- Hisabori-Wakabayashi Laboratory
- Hisabori Laboratory (Japanese)
- Toru Hisabori | Researcher Finder - Tokyo Tech STAR Search
- Ken-ichi Wakabayashi | Researcher Finder - Tokyo Tech STAR Search
- Laboratory for Chemistry and Life Science, Institute of Innovative Research
- Institute of Innovative Research (IIR)
- Department of Life Science and Technology, School of Life Science and Technology
- Okayama University
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Further Information
Professor Toru Hisabori
Institute of Innovative Research, Tokyo Institute of Technology
E-mail : thisabor@res.titech.ac.jp