Chemical Science and Engineering News
Keeping a Clean Path: Doubling the Capacity of Solid-State Lithium Batteries
Scientists at Tokyo Institute of Technology (Tokyo Tech), Tohoku University, National Institute of Advanced Industrial Science and Technology, and Nippon Institute of Technology, demonstrated by experiment that a clean electrolyte/electrode interface is key to realizing high-capacity solid-state lithium batteries. Their findings could pave the way for improved battery designs with increased capacity, stability, and safety for both mobile devices and electric vehicles.
Liquid lithium-ion batteries are everywhere, being found in the majority of everyday mobile devices. While they possess a fair share of advantages, liquid-based batteries carry notable risks as well. This has become clear to the public in recent years after reports of smartphones bursting into flames due to design errors that caused the battery's liquid electrolyte to leak and catch fire.
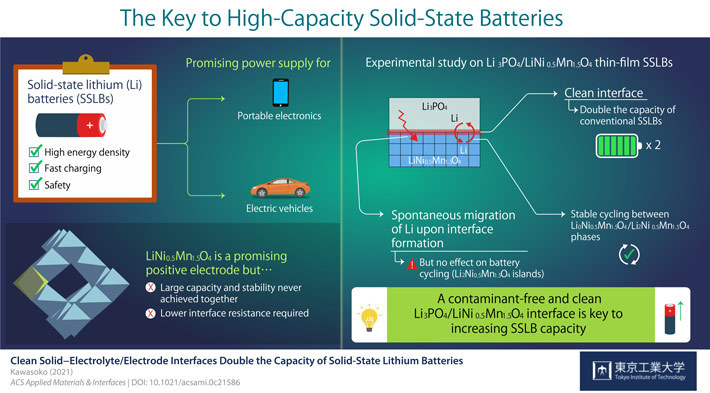
Other disadvantages such as fabrication cost, durability, and capacity, led scientists to look into a different technology: solid-state lithium batteries (SSLBs). SSLBs comprise solid electrodes and a solid electrolyte that exchange lithium (Li) ions during charging and discharging. Their higher energy density and safety make SSLBs very powerful sources.
However, there are still many technical challenges preventing SSLBs' commercialization. For the current study, researchers conducted a series of experiments and gained insight that could take SSLBs' performance to the next level. Professor Taro Hitosugi from Tokyo Tech, who led the study, explains their motivation: "LiNi0.5Mn1.5O4 (LNMO) is a promising material for the positive electrode of SSLBs because it can generate comparatively higher voltages. In this study, we showed battery operations at 2.9 and 4.7 V, and simultaneously achieved large capacity, stable cycling, and low resistance at the electrolyte/electrode interface."
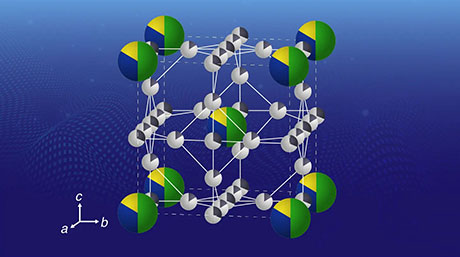
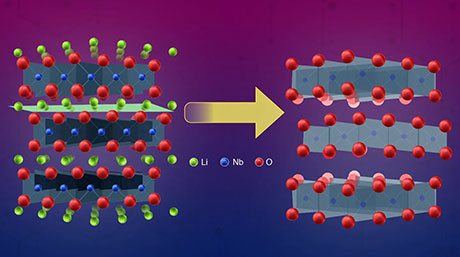
Previous studies had hinted that producing a clean electrolyte/electrode interface was essential to achieve low interface resistance and fast charging in LNMO-based SSLBs. Scientists also noted that Li ions spontaneously migrated from Li3PO4 (LPO) electrolyte to the LNMO layer upon fabrication, forming a Li2Ni0.5Mn1.5O4 (L2NMO) phase in LNMO with unknown distribution and impact on battery performance.
The team investigated what the L2NMO phase was like, analyzing the changes in crystalline structure between the Li0Ni0.5Mn1.5O4 (L0NMO) and L2NMO phases during charging and discharging. They also studied the initial distribution of L2NMO at clean LPO/LNMO interfaces fabricated in a vacuum, as well as the effect of electrode thickness.
Strikingly, the clean interface facilitated the intercalation and deintercalation of Li during charging and discharging of the SSLBs. As a result, the capacity of SSLBs with a clean interface was twice that of conventional LNMO-based batteries. Moreover, this study marked the first time stable reversible reactions were found between the L0NMO and L2NMO phases in SSLBs.
Assistant Professor Hideyuki Kawasoko of Tohoku University and lead author of the study remarked, "Our findings indicate that the formation of a contamination-free, clean LPO/LNMO interface is key to increasing the capacity of SSLBs while ensuring low interface resistance for fast charging."
Aside from mobile devices, SSLBs could find a home in electric cars, for which cost and battery durability act as major barriers for widespread commercialization. The results of this study provide important insight for future SSLB designs and pave the way for a transition away from fossil fuels and towards more ecofriendly ways of transportation. Keep an eye out for the advent of SSLBs!
- Reference
| Authors : | Hideyuki Kawasoko1,*, Tetsuroh Shirasawa2,*, Kazunori Nishio3, Ryota Shimizu3, Susumu Shiraki4, and Taro Hitosugi3 |
|---|---|
| Title of original paper : | Clean Solid−Electrolyte/Electrode Interfaces Double the Capacity of Solid-State Lithium Batteries |
| Journal : | ACS Applied Materials & Interfaces |
| DOI : | 10.1021/acsami.0c21586 |
| Affiliations : |
1 Department of Chemistry, Graduate School of Science, Tohoku University 2 National Metrology Institute of Japan, National Institute of Advanced Industrial Science and Technology (AIST) 3 School of Materials and Chemical Technology, Tokyo Institute of Technology 4 Department of Applied Chemistry, Nippon Institute of Technology |
- Fostering creativity in researchers: How Automation Can Revolutionize Materials Research | Tokyo Tech News
- 'Ironing' out the differences: Understanding superconductivity in ultrathin FeSe | Tokyo Tech News
- Small, fast, and highly energy-efficient memory device inspired by lithium-ion batteries | Tokyo Tech News
- Making it crystal clear: Crystallinity reduces resistance in all-solid-state batteries | Tokyo Tech News
- Expanding the limits of Li-ion batteries: Interface for all-solid-state batteries | Tokyo Tech News
- Toward the next generation of superconductors and lithium-ion batteries: Scanning the surface of lithium titanate | Tokyo Tech News
- Learning how to fine-tune nanofabrication | Tokyo Tech News
- Hitosugi & Shimizu Lab
- Researcher Profile | Tokyo Tech STAR Search - Taro Hitosugi
- Researcher Profile | Tokyo Tech STAR Search - Kazunori Nishio
- Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Department of Chemistry, Faculty of Science and Graduate School of Science, Tohoku University
- National Institute of Advanced Industrial Science and Technology (AIST)
- Nippon Institute of Technology
- Chemical Science and Engineering Graduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Latest Research News
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Professor Taro Hitosugi
School of Materials and Chemical Technology,
Tokyo Institute of Technology
Email hitosugi.t.aa@m.titech.ac.jp
Tel +81-3-5734-2636