Chemical Science and Engineering News
Synthesizing a Superatom: Opening Doors to their Use as Substitutes for Elemental Atoms
Scientists at Tokyo Institute of Technology (Tokyo Tech) and ERATO Japan Science and Technology have demonstrated how superatoms of a desired valency, stability, and volume can be synthesized in a solution medium by altering the number of atoms in a cluster structure. This is an important step in realizing the practical application of superatom clusters as substitutes for elements in chemical reactions.
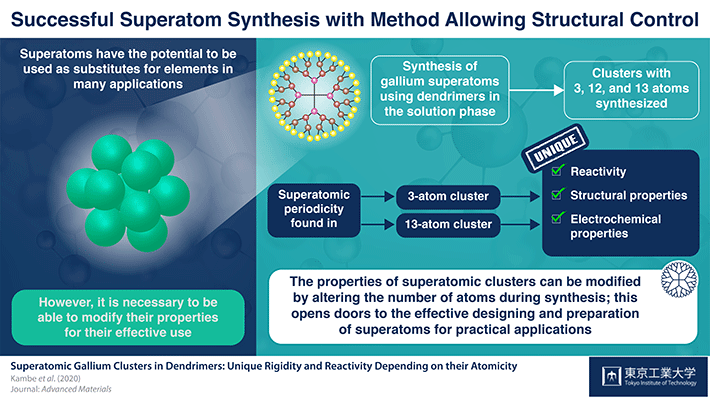
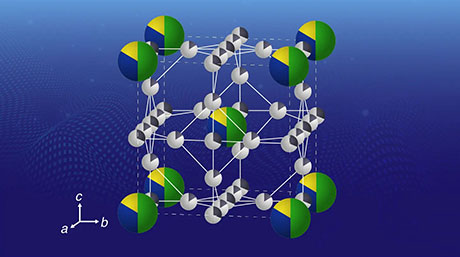
Figure 1. Schematic illustration of superatomic clusters with 3, 12 and 13 atoms.
Superatom is a name given to a cluster of atoms that seem to exhibit properties similar to elemental atoms. Scientists have shown particular interest in superatomic structures, since they can be linked with atoms to produce molecules, and potentially be used to substitute certain elements in many applications.
But for superatoms to be effectively utilized, they must be specially tailored to resemble the characteristics of the corresponding elements. This transformation depends on the specific combination of electrons used. For example, when an aluminum atom with 3 valence electrons (outer shell electrons that can contribute to the formation of chemical bonds) is added to the superatom of aluminium-13, the properties change to those of a superatom of aluminium-14. Due to this modifiability of superatoms, investigating them and understanding them further is important. But previous research has been mainly theoretical, and largely focused on single clusters. Research has also not been able to synthesize superatomic clusters with sufficient volume and stability for practical application.
In a recent study, scientists from Tokyo Tech and ERATO Japan Science and Technology, led by Dr Tetsuya Kambe and Prof Kimihisa Yamamoto, fabricated clusters of the element gallium (Ga) in solution to demonstrate the effects of changing the number of atoms in a cluster on the properties of the cluster. The team synthesized Ga clusters of 3, 12, 13 and other numbers of atoms using a specialized superatom synthesizer. To characterize and analyze the structural differences among the synthesized clusters, transmission electron microscopic images were captured and calculations were performed using computation tools.
The mass spectrometry revealed that the 13- and 3-atom clusters had superatomic periodicity. The 13-atom cluster differed from the other clusters structurally and electrochemically. But the 3-atom cluster with hydrogen (Ga3H2) was reduced to Ga3H2- and was not detected, suggesting a low stability of this cluster when synthesized in the solution medium.
The ability to alter the clusters reinforces the concept that structural change can be induced in superatoms. Describing the implications of their findings, the scientists explain: "These series of results demonstrate that it is possible to change the valence electrons in superatomic clusters in solution by controlling the number of constituent atoms. This in turn enables the designing and preparation of superatoms."
This study paves the way for future research to investigate the use of superatoms as substitutes for elements. As Dr Kambe, Prof Yamamoto and team reiterate, "the superatom reveals an attractive strategy for creating new building blocks through the use of cluster structures."
- Reference
| Authors : | Tetsuya Kambe1,2, Aiko Watanabe1, Meijia Li1, Takamasa Tsukamoto1,2, Takane Imaoka1,2, and Kimihisa Yamamoto*1,2 *Corresponding author's email: yamamoto@res.titech.ac.jp |
|---|---|
| Title of original paper : | Superatomic Gallium Clusters in Dendrimers: Unique Rigidity and Reactivity Depending on their Atomicity |
| Journal : | Advanced Materials |
| DOI : | 10.1002/adma.201907167 |
| Affiliations : |
1 Laboratory for Chemistry and Life Science, Tokyo Institute of Technology 2 ERATO Japan Science and Technology (JST) |
- The power of going small: Copper oxide subnanoparticle catalysts prove most superior | Tokyo Tech News
- Discovery of periodic tables for molecules | Tokyo Tech News
- Can't get thinner than this: synthesis of atomically flat boron sheets | Tokyo Tech News
- Metallic nano-particles light up another path towards eco-friendly catalysts | Tokyo Tech News
- Breakthrough in blending metals: Precise control of multimetallic one-nanometer cluster formation achieved | Tokyo Tech News
- How a tetrahedral substance can be more symmetrical than a spherical atom | Tokyo Tech News
- One-Nanometer Trimetallic Alloy Particles Created | Tokyo Tech News
- Controllable light-emitting materials to advance light sensing and nano-medicine | Tokyo Tech News
- A step forward for fuel cell technology | Tokyo Tech News
- YAMAMOTO-IMAOKA Group
- Researcher Profile | Tokyo Tech STAR Search - Tetsuya Kambe
- Researcher Profile | Tokyo Tech STAR Search - Takamasa Tsukamoto
- Researcher Profile | Tokyo Tech STAR Search - Takane Imaoka
- Researcher Profile | Tokyo Tech STAR Search - Kimihisa Yamamoto
- Laboratory for Chemistry and Life Science, Institute of Innovative Research
- Institute of Innovative Research (IIR)
- Yamamoto Atom Hybrid Project | JST ERATO
- ERATO Japan Science and Technology (JST)
- Latest Research News
Further Information
Assistant Professor Tetsuya Kambe
Institute of Innovative Research, Tokyo Institute of Technology
Email kambe.t.aa@m.titech.ac.jp
Tel +81-45-924-5259