Chemical Science and Engineering News
Nitrous oxide emissions set to rise in the western North Pacific Ocean
Key point
- Response of N2O production process to ocean acidification is verified
- In the western North Pacific, ocean acidification would enhance the production of N2O
- The new finding refine the prediction of future climate change
Summary
The acidification of the western North Pacific Ocean is increasing the natural production rate of N2O, an ozone-depleting greenhouse gas. That's the finding of a study carried out jointly by scientists at EPFL, Switzerland, Tokyo Institute of Technology (Prof. Naohiro Yoshida and Assoc. Prof. Sakae Toyoda) and Japan Agency for Marine-Earth Science and Technology and appearing recently in Nature Climate Change. The research team conducted on-board seawater incubation experiments in the western North Pacific Ocean, and discovered that the water's lower pH is causing a significant increase in N2O production. Although it was thought that acidification decreases the natural production rate of N2O, the new research has discovered that the process appears to work the other way around. The study has just been published in Nature Climate Change.
Background
Today's rising CO2 emissions are not only causing the global warming but also changing oceans' pH levels, making them more acidic. We can already see the harmful effects in the coral reefs. Yet other chemical processes – whose environmental impact is not fully known – are also being affected. A study by Beman et al. published in the Proceedings of the National Academy of Sciences in 2011 suggested that ocean acidification is lowering the rate at which nitrous oxide (N2O), an ozone-depleting greenhouse gas (also known as laughing gas), is being produced naturally. Based on this study, it was thought that acidification decreases the natural production rate of N2O.
Research outcome
The research team collected samples at five different sites off the coast of Japan, from the subarctic region to the subtropical region. Then they lowered the samples' pH levels, triggering the natural process whereby microbes in the water convert ammonium into nitrate, which generates N2O as a by-product. Acidification did not suppress the N2O production at all the sites. At the subarctic sites, in particular, the samples showed a decrease in the ammonium-to-nitrate conversion rate, as in the PNAS study, but also an increase in N2O production. Moreover, they concluded that if pH levels keep falling at the current rate, or 0.0051 units/year – assuming there is no decrease in CO2 emissions – the N2O production rate in that part of the Pacific could rise by 190% to 500% by 2100.
Future development
The study revealed that N2O emission from the western North Pacific Ocean would not decrease, but increase by ocean acidification. However, there are production and consumption processes of N2O other than nitrification tested in this work, and dominant process would be different among oceanic regions. Increase in atmospheric N2O concentration accelerates global warming and delays the recovery of ozone layer that was damaged by chlorofluorocarbons. Additional research is needed to see the response of other N2O producing/consuming processes to ocean acidification as well as to study on other oceanic regions.
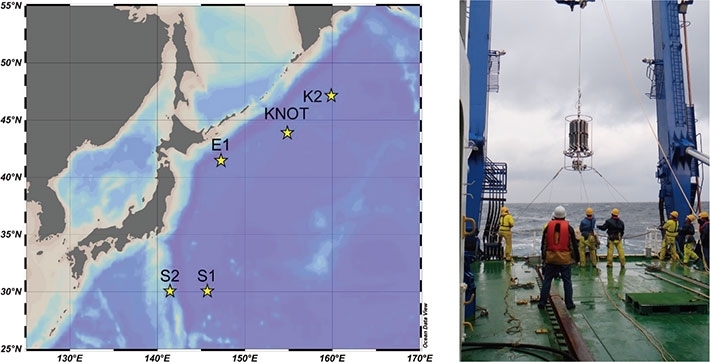
- Fig.1. Map of study sites (stars) and picture of seawater sampling at subarctic station KNOT. Water sampler was casted in the sea from the research ship and seawater of active at 100-200 m depth, where microbial nitrification is active, was collected.

- Fig.2. Picture of onboard seawater incubation experiment. 15N-labeled tracer was added to the water sample, and pH was decreased by addition of hydrochloric acid. The manipulated samples are kept in dark at the temperatures same as those at the sampling depth.
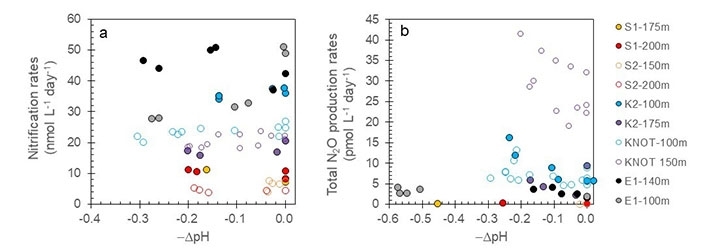
- Fig.3. Results of acidification experiments. Horizontal axis shows decrease of pH whereas vertical axis shows rate of nitrification or N2O production. Symbols with warm and cold colors respectively denote results for subtropiccal and subarctic sites. Decrease in pH is accompanied by decrease in nitrification rate and increase in N2O production rate.
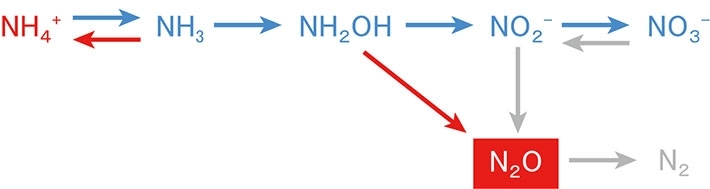
- Fig.4. Production and consumption processes of N2O. Red and blue arrows show nitrification, and gray arrows show nitrifier-dnitrification and denitrification. In the region studied in this work, N2O is mainly produced by nitrification and denitrification. Red-colored substance and arrows are respectively increased and enhanced by ocean acidification whereas blue-colored ones are decreased or suppressed. The present study revealed that the process from NH2OH to N2O is enhanced by ocean acidification.
Acknowledgement
This project was financially supported by Kakenhi (JP23224013, JP15H05822, JP15H05471, and 17H06105) of the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and the Swiss National Science Foundation (PBNEP2-142954). It utilized samples collected in the research cruises MR13-04, KS-16-8, and YK16-16 of JAMSTEC.
- Reference
| Authors : | Florian Breider 1,2, Chisato Yoshikawa 3, Akiko Makabe 4, Sakae Toyoda 5, Masahide Wakita 6, Yohei Matsui 7, Shinsuke Kawagucci 4, Tetsuichi Fujiki 6, Naomi Harada 6, and Naohiro Yoshida1,5,8 |
|---|---|
| Title of original paper : | Response of N2O production rate to ocean acidification in the western North Pacific |
| Journal : | Nature Climate Change |
| DOI : | 10.1038/s41558-019-0605-7 |
| Affiliations : |
1Department of Environmental Chemistry and Engineering, Tokyo Institute of Technology, Nagatsuta 4259, Midori-ku, Yokohama, 226-8503 Kanagawa, Japan 2Institute of Environmental Engineering, Ecole Polytechnique Fédérale de Lausanne - EPFL, Station 2, CH-1015 Lausanne, Switzerland 3Research Institute for Marine Resources Utilization, Japan Agency of Marine Earth Science and Technology, 2-15 Natsushima-cho, Yokosuka-city 237-0061, Japan 4Institute for Extra-cutting-edge Science and Technology Avant-garde Research (X-star), Japan Agency of Marine Earth Science and Technology, 2-15 Natsushima-cho, Yokosuka-city 237-0061, Japan 5School of Materials and Chemical Technology, Tokyo Institute of Technology, Nagatsuta 4259, Midori-ku, Yokohama, 226-8503 Kanagawa, Japan 6Research Institute for Global Change (RIGC), Japan Agency of Marine Earth Science and Technology,2-15 Natsushima-cho, Yokosuka-city 237-0061, Japan 7Atmosphere and Ocean Research Institute, The University of Tokyo, 5-1-5, Kashiwanoha, Kashiwa-shi, Chiba 277-8564 Japan 8Earth-Life Science Institute, Tokyo Institute of Technology, Meguro, 152-8551 Tokyo, Japan |
- Electricity-Driven Undersea Reactions May have Been Important for the Emergence of Life | Tokyo Tech News
- Tokyo Tech Scientists Discover Deep Microbes' Key Contribution to Earth's Carbon Cycle | Tokyo Tech News
- Isotopic makeup of atmospheric sulfate and nitrate | Tokyo Tech News
- Organic chemical origins in hydrothermal systems | Tokyo Tech News
- Yoshida / Toyoda / Yamada's Laboratory
- Researcher Profile | Tokyo Tech STAR Search - Sakae Toyoda
- Researcher Profile | Tokyo Tech STAR Search - Naohiro Yoshida
- ELSI
- EPFL
- Atmosphere and Ocean Research Institute, The University of Tokyo
- JAMSTEC - Japan Agency for Marine-Earth Science and Technology
- Latest Research News
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Associate Professor Sakae Toyoda
School of Materials and Chemical Technology,
Tokyo Institute of Technology