Life Science and Technology News
New Mechanism Uncovered for the Reduction of Emu Wings
The Lack of Muscle at the Distal Wings Leads to Bone Reduction and Asymmetry
Researchers have uncovered a fascinating mechanism behind the reduction and asymmetry of emu wing bones. The wings not only show significant shortening, but the skeletal elements also fuse asymmetrically, a phenomenon traced back to the absence of muscle formation in the distal regions of the wings. During development, this lack of muscle leads to insufficient mechanical stress, which is crucial for proper bone formation. The team identified muscle progenitor cells with a unique dual identity, combining characteristics of both somite[1]-derived myogenic and lateral plate mesoderm[2] cells. These cells undergo cell death during muscle development, preventing the formation of distal muscles. The study highlights how differences in embryonic and fetal movement may play a pivotal role in driving morphological evolution, shedding light on the complex developmental processes that shape skeletal structures.
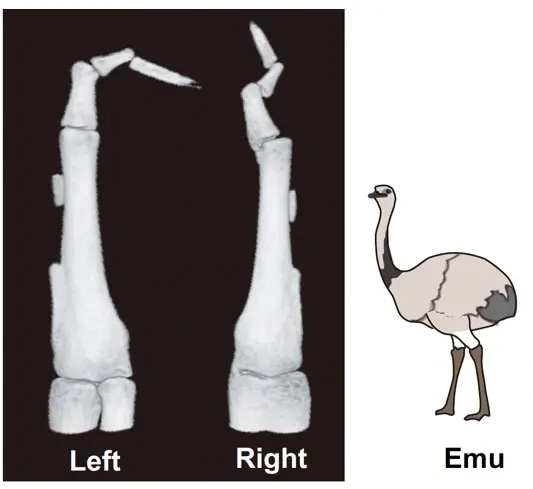
Figure 1. Emu wings exhibit a left-right asymmetry in the reduction and fusion of skeletal elements.
Professor Mikiko Tanaka from the School of Life Science and Technology at Institute of Science Tokyo , along with her team, including Eriko Tsuboi and Ingrid Rosenburg Cordeiro (both former graduate students at Tokyo Institute of Technology), and current graduate student Satomi Ono, in collaboration with Professor Shuji Shigenobu of the National Institute for Basic Biology, Professor Guojun Sheng of Kumamoto University, and Professor Masataka Okabe of Jikei University School of Medicine, have uncovered a novel mechanism underlying the skeletal reduction and asymmetry of emu wings. Their research reveals that the absence of distal muscle formation results in a lack of mechanostress during development, leading to the observed bone abnormalities. Furthermore, the study identified the presence of muscle progenitor cells with a dual identity, which undergo cell death during the differentiation into muscle fibers, thereby preventing proper muscle formation. This research suggests that variations in embryonic and fetal movement could play a significant role in shaping the body parts throughout evolution. These findings will be published in Nature Communications on September 19, 2024.
Background
The emu is a flightless bird with wings that have undergone significant reduction. Despite this, the precise mechanisms behind the morphological changes in their wings have remained largely unknown. In this study, the research team demonstrated that the skeletal reduction in emu wings is not only characterized by shortening but also by an asymmetric fusion of bones. They identified that these skeletal abnormalities are caused by a lack of muscle formation at the distal wings, which results in insufficient movement during development – which is required for the shaping the embryonic and fetal skeleton. Additionally, the study discovered that the presence of muscle progenitor cells in emu wings that exhibit a dual identity, combining features of both somite-derived muscle progenitor cells and lateral plate mesoderm cells. These cells undergo cell death during the differentiation into muscle fibers, leading to a failure in muscle formation. The findings suggest that differences in embryonic and fetal movement can significantly influence morphological evolution.
Research Findings
The research team confirmed that the bones of emu wings are not only shortened but also show significant variation in pattern and length between individuals, and even between the left and right wings of the same individual (Figure 1). This distinctive skeletal pattern is linked to the lack of muscle formation at the distal region of their wings, which leads to inadequate mechanical stress during bone development. The study also revealed that the presence of muscle progenitor cells with a dual identity—combining characteristics of both somite-derived muscle progenitor cells and lateral plate mesoderm cells—results in cell death during the muscle fiber formation (Figure 2). This cell death disrupts the development of the wing's muscle structure, leading to immobilization and subsequent skeletal abnormalities.
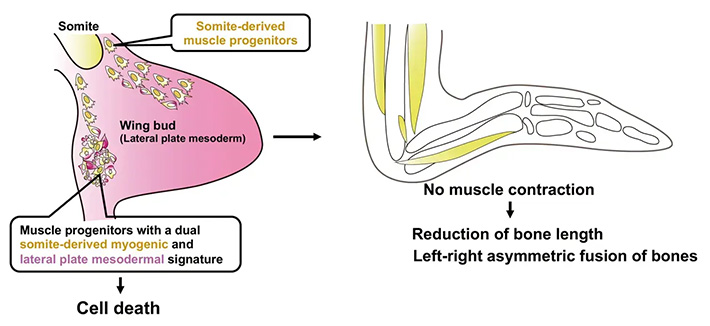
Figure 2. Proposed Model: Emu wing buds contain muscle progenitor cells with dual somite-derived myogenic and lateral plate mesodermal signature. These cells undergo cell death, which hinders the normal development of wing muscles. As a result, the lack of mechanical stress from muscle contractions leads to shortened bones and asymmetric fusion of skeletal elements.
Societal Impact
This study highlights the crucial role that embryonic and fetal movement plays not only in the elongation of skeletal elements but also in the symmetrical patterning of bones. The findings underscore the significant impact that insufficient embryonic movement, particularly in cases of muscle formation defects like those observed in emus, can have on skeletal evolution. The research suggests that environmental factors influencing embryonic and fetal movement could have far-reaching effects on morphological evolution and diversification.
Future Directions
This research has demonstrated the profound impact that embryonic and fetal movement can have on the evolution of skeletal morphology. Moving forward, the team plans to investigate how variations in embryonic and fetal movement might influence skeletal evolution across vertebrates. This groundbreaking study opens new avenues for understanding the role of environmental factors in shaping the evolution of morphology through their effects on embryonic and fetal movement.
- Funding
This work was supported by JSPS KAKENHI Grant Numbers JP20H03301, and JP17KT0106, MEXT KEKNHI JP18H04818, NIBB Collaborative Research Program (21-357), Astellas Foundation for Research on Metabolic Disorders, Mitsubishi Foundation and Yamada Science Foundation to M.T.
- Terms
[1] Somite: Block-like structures found in the embryos of developing vertebrates. Somites give rise to cells that form muscles, bones, and the dermis of the skin. Typically, the muscles of the limbs originates from somites.
[2] Lateral Plate Mesoderm: A portion of the mesoderm located on the outer side of the embyo, responsible for forming the limb buds, body wall, heart, and blood vessels.
- Reference
| Authors: | Eriko Tsuboi1†, Satomi F Ono1†, Ingrid Rosenburg Cordeiro1‡†, Reiko Yu1, Toru Kawanishi1, Makoto Koizumi2, Shuji Shigenobu3, Guojun Sheng4, Masataka Okabe5, and Mikiko Tanaka1* †These authors contributed equally to this work. |
|---|---|
| Title: | Immobilization secondary to cell death of muscle precursors with a dual transcriptional signature contributes to the emu wing skeletal pattern |
| Journal: | Nature Communications |
| Affiliations: | 1 School of Life Science and Technology, Tokyo Institute of Technology, Japan 2 Laboratory Animal Facilities, The Jikei University School of Medicine, Japan 3 Trans-Omics Facility, National Institute for Basic Biology, Japan 4 International Research Center for Medical Sciences, Kumamoto University, Japan 5 Department of Anatomy, The Jikei University School of Medicine, Japan ‡ Present address: Department of Cell and Molecular Biology, Karolinska Institutet, Sweden |
| DOI: | 10.1038/s41467-024-52203-x |
- Oxygen shapes arms and legs: origins of a new developmental mechanism called "interdigital cell death"|Life Science and Technology News
- Basis of Development of Vertebrate Limb Muscles has been established in Cartilaginous Fishes|Life Science and Technology News
- Key genetic event underlying fin-to-limb evolution | Tokyo Tech
- Mechanism determining survival-and-death fate of cells in limb formation clarified | Tokyo Tech
- From fins to limbs: Unravelling the mystery of evolutionary morphology | Tokyo Tech
- Mikiko Tanaka | Researcher Finder - Science Tokyo STAR Search
- Mikiko Tanaka Laboratory
- [Labs spotlight] Mikiko Tanaka Laboratory | Life Science and Technology News
- Department of Life Science and Technology, School of Life Science and Technology
- School of Life Science and Technology
- National Institute for Basic Biology
- Kumamoto University
- The Jikei University School of Medicine
- Research
Further Information
Professor Mikiko Tanaka
School of Life Science and Technology, Institute of Science Tokyo
Tel +81-45-924-5722
E-mail mitanaka@bio.titech.ac.jp