Life Science and Technology News
【Labs spotlight】 Nakamura and Okada Laboratory
Control of Biofunctions for Drug Development

Areas of Supervision
Primary/Science and Technology for health Care and Medicine
Secondary/Life Science and Technology, Human Centered Science and Biomedical Engineering
Professor Hiroyuki Nakamura![]()

Areas of Supervision
Primary/Science and Technology for health Care and Medicine
Secondary/Life Science and Technology, Human Centered Science and Biomedical Engineering
Associate Professor Satoshi Okada![]()
| Degree | Professor Hiroyuki Nakamura : PhD 1996, Tohoku University Associate Professor Satoshi Okada : PhD 2012, Osaka University |
|---|---|
| Areas of Research | Professor Hiroyuki Nakamura : Synthetic Organic Chemistry, Medicinal Chemistry, Chemical Biology, Boron Neutron Capture Therapy Associate Professor Satoshi Okada : Bioengineering, Molecular Imaging, Chemical Biology |
| Keywords | Professor Hiroyuki Nakamura : Protein Kinases Inhibitors, Drug Delivery System, New Synthetic Processes, Cancer Therapy Associate Professor Satoshi Okada : Magnetic Resonance Imaging, Probes, Biomaterials, Neurotransmitters |
| Website | Nakamura and Okada Laboratory |
Research interest
Our research interests are focused on discovery of drug candidates for anticancer agents, development of novel protein labeling systems, development of efficient boron delivery system in neutron capture therapy of cancer, and development of magnetic probes for imaging of biological functions. Our research is directed to interdisciplinary area based on organic synthetic technology to explore and control vital function for innovative drug discovery.
(1) Synthesis of Novel Basic Skeletons of Bioactive Compounds
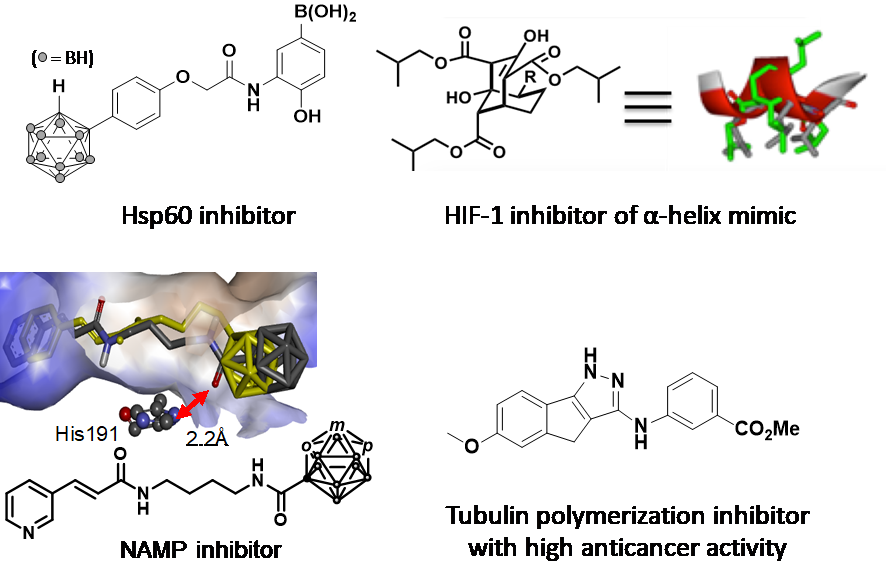
Fig. 1 The synthesized bioactive compounds.
Development of core skeletons of bioactive compounds is important research in drug discovery. We design and synthesize novel skeletons based on boron and three dimensional small molecules targeting protein-protein interaction. The biological activities of the synthesized molecules are estimated by ourselves and fed back to the molecular design.
(2) Development of Next-Generation Boron Nanocarriers for Boron Neutron Capture Therapy (BNCT)
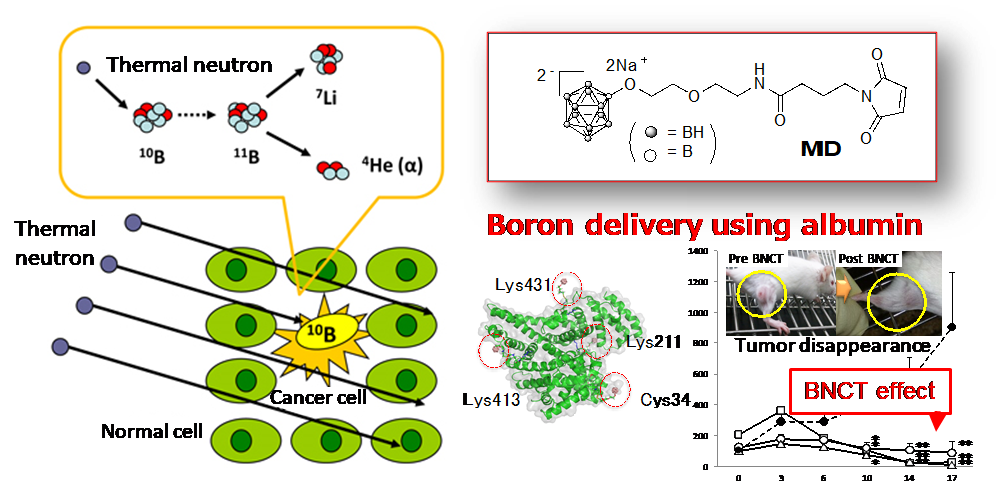
Fig. 2 Schematic image of BNCT for cancer therapy.
BNCT is a less invasive cancer therapy based on a reaction of thermal neutrons with boron-10 of agents. In order to increase the efficacy of therapeutic effect, we develop a next-generation nanocarrier for the efficient delivery of boron compounds to cancer tissues.
(3) Development of Magnetic Probes for Imaging and Controlling of Biological Functions
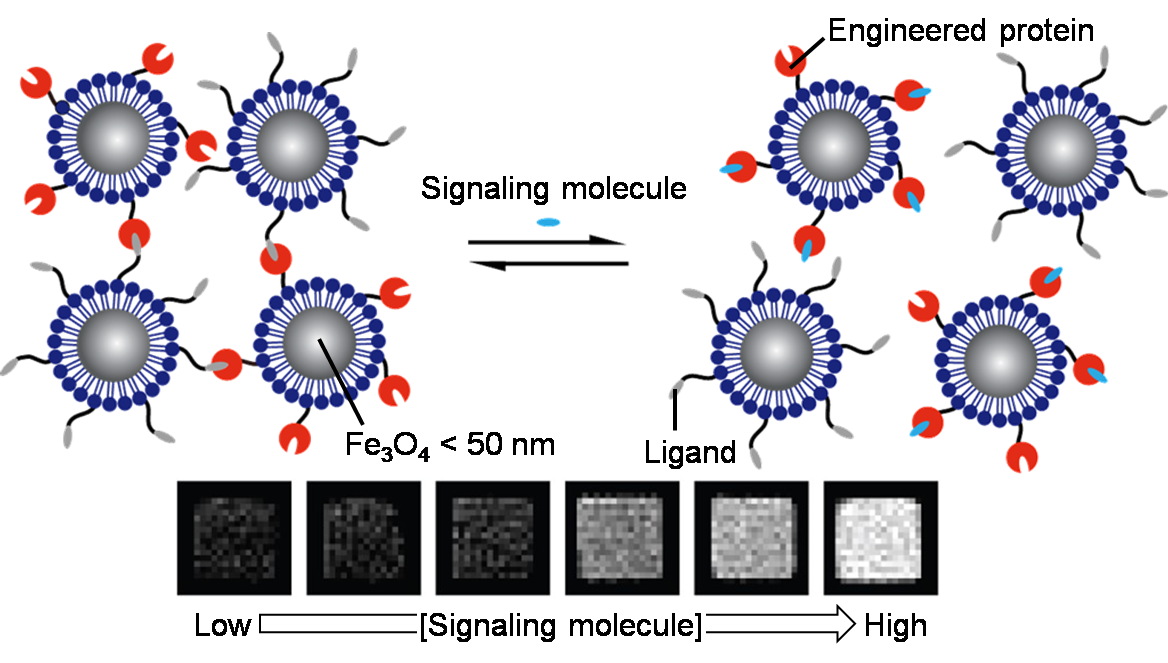
Fig. 3 MRI nanoprobes for imaging of signaling molecules.
The imaging modalities using magnetic field is a powerful tool to observe a wide region of the body, although their molecular specificity is inferior to optical modalities. To address this issue, we develop magnetic probes that convert a specific signaling molecule to MRI contrast.
(4) Development of Protein Labeling Systems Using a Photocatalyst
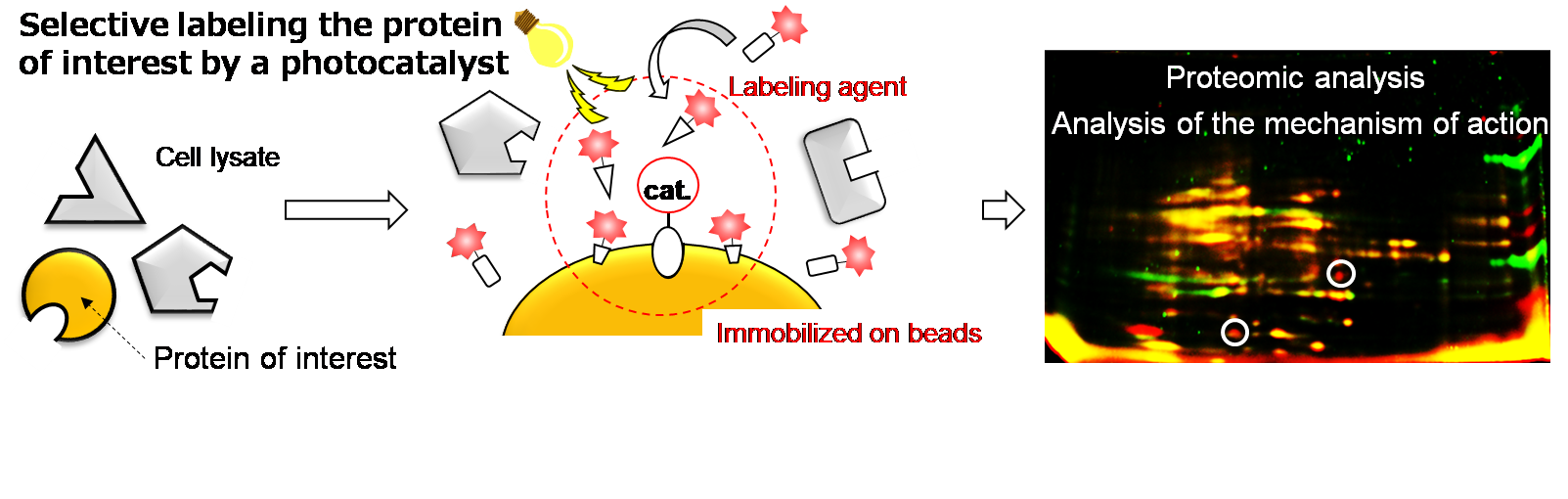
Fig. 4 Selective protein labeling using a photoredox catalyst.
Protein labeling is a useful technology to identify the target protein of an inhibitor and the mode of action. We utilize a photoredox catalyst to label the proteins that are unlikely to be identified by the current technology
Research findings
Selected publications
Our publication list is available from our web site![]() .
.
- [1] "Site-Selective Protein Chemical Modification in Exposed Tyrosine Residues Using Tyrosine Click Reaction" S. Sato, M. Matsumura, T. Kadonosono, S. Abe, T. Ueno, H. Ueda, H. Nakamura, Bioconjugate Chem. accepted DOI: 10.1021/acs.bioconjchem.0c00120
 (2020).
(2020). - [2] "N-Methylated Peptide Synthesis through Acyl N-Methylimidazolium Cation Generation Accelerated by a Bronsted Acid” Y. Otake, Y. Shibata, Y. Hayashi, S. Kawauchi, H. Nakamura, S. Fuse, Angew. Chem. Int. Ed. accepted DOI: 10.1002/anie.202002106

- [3] "Rhodium(III)-Catalysed Carboxylate-Directed C-H Functionalizations of Isoxazoles with Alkynes" S. Yugandar, H. Nakamura, Chem. Commun. 55 (58), 8382?8385 (2019).
- [4] "Neurotransmitter-Responsive Nanosensors for T2-Weighted Magnetic Resonance Imaging" V. Hsieh, S. Okada, H. Wei, I. Garcia-Alvarez, A. Barandov, S. R. Alvarado, R. Ohlendorf, J. Fan, A. Ortega, A. Jasanoff, J. Am. Chem. Soc. 141 (40), 15751?15754 (2019).
- [5] "Gold(I)-Catalyzed Intramolecular SEAr Reaction: Efficient Synthesis of Isoxazole-Containing Fused Heterocycles" T. Morita, S. Fukuhara, S. Fuse, H. Nakamura, Org. Lett. 20 (2), 433?436 (2018).
- [6] "Calcium-Dependent Molecular FMRI Using a Magnetic Nanosensor" S. Okada, B. B. Bartelle, N. Li, V. Breton-Provencher, J. J. Lee, E. Rodriguez, J. Melican, M. Sur, A. Jasanoff, Nat. Nanotech. 13 (6), 473?477 (2018).
- [7] "Horseradish-Peroxidase-Catalyzed Tyrosine Click Reaction" S. Sato, K. Nakamura, H. Nakamura, ChemBioChem. 18 (5), 475?478 (2017).
- [8] "The Life of Pi Star: Exploring the Exciting and Forbidden Worlds of the Benzophenone Photophore" G. Dorman, H. Nakamura, A. Pulsipher, G. D. Prestwich, Chem. Rev. 116 (24), 15284?15398 (2016).
- [9] "Total Synthesis of Feglymycin based on a Linear/Convergent Hybrid Approach using Micro-flow Amide Bond Formation" S. Fuse, Y. Mifune, H. Nakamura, H. Tanaka, Nat. Commun. 7, 13491 (2016).
- [10] "Generation of 4-Isoxazolyl Anion Species: Facile Access to 4-Substituted Isoxazoles" T. Morita, S. Fuse, H. Nakamura, Angew. Chem. Int. Ed. 55 (43), 13580-13584 (2016).
- [11] "Maleimide-Functionalized closo-Dodecaborate Albumin Conjugates (MID-AC): The Unique Ligation at both Cysteine and Lysine Residues Enabling to Efficient Boron Delivery to Tumor for Neutron Capture Therapy" S. Kikuchi, D. Kanoh, S. Sato, Y. Sakurai, M. Suzuki, H. Nakamura J. Control. Release (237), 160?167 (2016).
- [12] "Synthesis of 2-Indolyltetrahydroquinolines by Zinc(II)-Catalyzed Intramolecular Hydroarylation-Redox Cross-Dehydrogenative Coupling of N-Propargylanilines with Indoles" G. Li, H. Nakamura, Angew. Chem. Int. Ed. 55 (23), 6758-6761 (2016).
- [13] "Hypoxia-Inducible Factor Inhibitors: A Patent Review (2011-2015)" S. Ban, Y. Uto, M. Won, H. Nakamura Exp. Opin. Ther. Pat. 26 (3), 309?322 (2016).
- [14] "Tyrosine-Specific Chemical Modification with in situ Hemin-Activated Luminol Derivatives" S. Sato, K. Nakamura, H. Nakamura, ACS Chem. Biol. 10 (11), 2633-2640 (2015).
- [15] "Methyl 3-((6-methoxy-1,4-dihydroindeno[1,2-c]pyrazol-3-yl)amino) benzoate (GN39482) as a Tubulin Polymerization Inhibitor Identified by MorphoBase and ChemProteoBase Profiling Methods" H. Minegishi, Y. Futamura, S. Fukashiro, M. Muroi, M. Kawatani, H. Osada, H. Nakamura, J. Med. Chem. 58 (10), 4230-4241 (2015).
- [16] "Boron-Based Drug Design" H. S. Ban and H. Nakamura, Chem. Rec. 15 (3), 616-635 (2015)
- [17] "Ligand-directed Selective Protein Modification Based on Local Single Electron Transfer Catalysis" S. Sato, H. Nakamura, Angew. Chem. Int. Ed. 52 (33) 8681-8684 (2013).
- [18] "Zinc(II)-Catalyzed Redox Cross-Dehydrogenative Coupling of Propargylic Amines and Terminal Alkynes for Synthesis of N-Tethered 1,6-Enynes" T. Sugiishi, H. Nakamura, J. Am. Chem. Soc. 134 (5) 2504-2507 (2012).
- [19] "Identification of HSP60 as a Primary Target of ortho-Carboranylphenoxyacetanilide, an HIF-1α Inhibitor" H. S. Ban, K. Shimizu, H. Minegishi, H. Nakamura, J. Am. Chem. Soc. 132 (34), 11870-11871 (2010).
- [20] “Copper(I)-Catalyzed Substitution Reactions of Propargylic Amines: Importance of Csp?Csp3 Bond Cleavage in Generation of Iminium Intermediates” T. Sugiishi, A. Kimura, H. Nakamura, J. Am. Chem. Soc. 132, 5332-5333 (2010).
- Research Laboratories and Subjects
- Boron carrier for targeted tumour therapy | Life Science and Technology News
- Laboratory for Chemistry and Life Science Institute of Innovative Research
Contact
Professor Hiroyuki Nakamura
Room 914, R1 building, Suzukakedai campus
E-mail : hiro@res.titech.ac.jp
Associate Professor Satoshi Okada
Room 913, R1 building, Suzukakedai campus
E-mail : sokada@res.titech.ac.jp
*Find more about the lab and the latest activities at the lab site![]() .
.
*May 1, 2025:Some of the content has been updated with the latest information.