Chemical Science and Engineering News
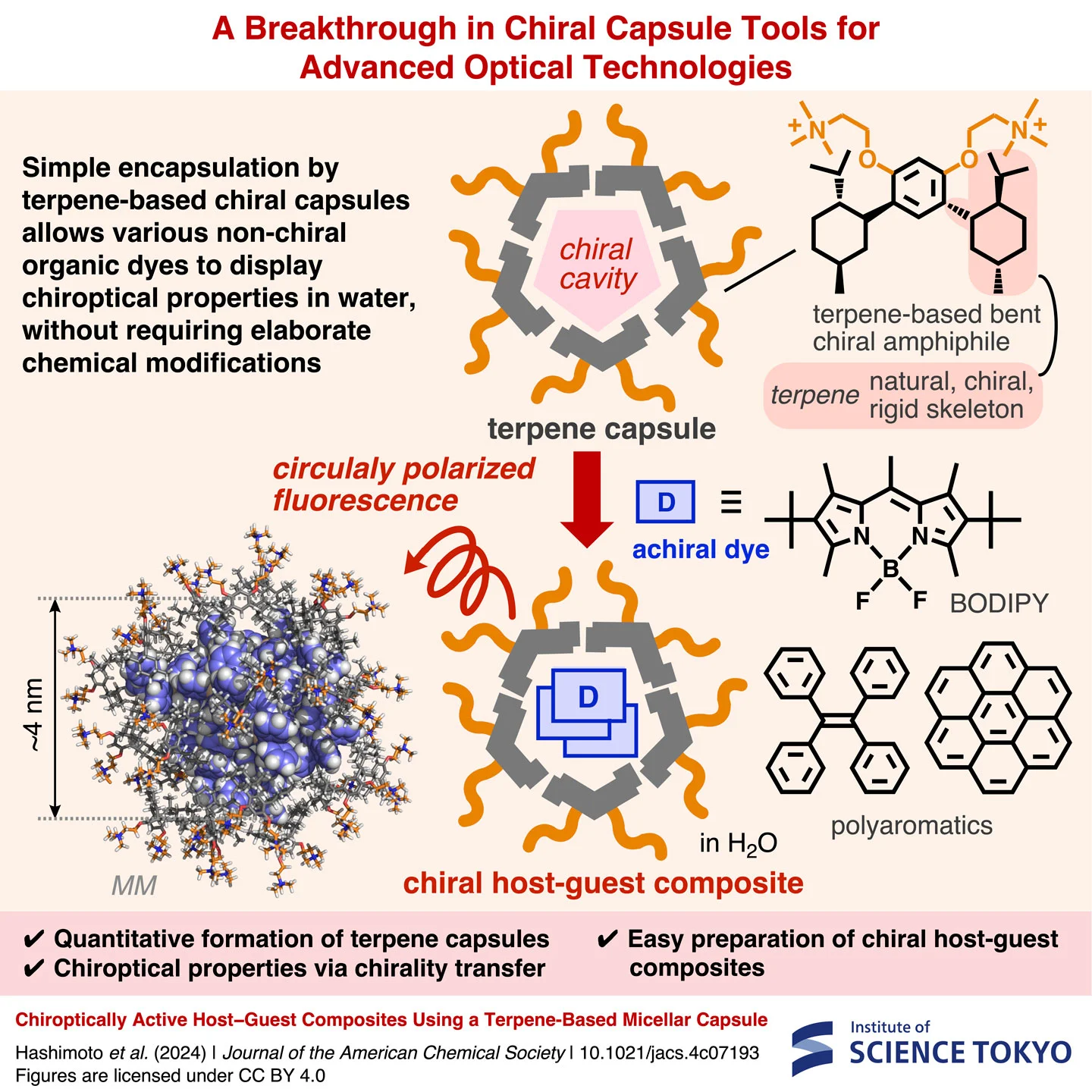
A Breakthrough in Chiral Capsule Tools for Advanced Optical Technologies
Simple encapsulation by terpene-based chiral capsules allows various non-chiral organic dyes to display chiroptical properties in water, without requiring elaborate chemical modifications, report scientists from Science Tokyo. The present molecular tools could pave the way to advanced optical technologies, spanning the fields of digital storage, biomedicine, and catalysis, to name a few.
Chirality is an essential property in biology; the molecular building blocks of some of the most important biological structures, including DNA and proteins, are chiral. When a molecule is said to be chiral, it means that it is distinguishable from its mirror image, much like how one’s left hand cannot be superposed onto the right hand. Interestingly, chirality can also affect how a molecule or a molecular aggregate emits or responds to light, especially circularly polarized light.
With the rise of advanced optical technologies, such 'chiroptically active' components could find applications in modern displays, optical storage systems, analytical tools, and biomedicine. Thus, scientists have been exploring innovative ways of making well-established non-chiral dyes behave like chiroptically active molecules. Through a process known as chirality transfer, dyes encapsulated in cavities made from chiral molecules can acquire chiroptical properties. However, the synthesis of usable chiral capsules has proven challenging, with existing designs being complex and inflexible.
In a recent study published in the Journal of the American Chemical Society![]() , a research team including Assistant Professor Yuya Tanaka and Yoshihisa Hashimoto from Institute of Science Tokyo has developed a new, simpler solution. They created novel chiral capsules based on terpenes, a class of natural compounds from plants. The terpene-based capsules offer an efficient and flexible way to transfer chirality to various non-chiral dyes in the cavity.
, a research team including Assistant Professor Yuya Tanaka and Yoshihisa Hashimoto from Institute of Science Tokyo has developed a new, simpler solution. They created novel chiral capsules based on terpenes, a class of natural compounds from plants. The terpene-based capsules offer an efficient and flexible way to transfer chirality to various non-chiral dyes in the cavity.
The present chiral capsules are composed of terpene-based bent amphiphilic molecules, obtained through a series of chemical modifications on menthol. The chiral amphiphiles, due to their hydrophilic and hydrophobic frameworks, spontaneously form globular structures called terpene capsules when mixed with water. When a non-chiral dye is mixed with the amphiphiles, a host–guest composite structure is formed, in which the dyes are accommodated in the chiral cavity.
Through extensive experiments, the researchers showed that chirality transfer occurred between the capsule and several types of non-chiral dyes. For example, fluorescent dyes, including polyaromatic and BODIPY compounds, exhibited strongly induced circular dichroism and circularly polarized fluorescence. Unlike previously reported chiral hosts, the present structure was highly versatile and easy to produce, as Dr. Tanaka remarks: "Our terpene capsules facilitate the easy preparation of well-defined host−guest composites with tunable chiroptical properties through simple uptake of various non-chiral dyes, unlike the majority of previously reported hosts with rigid chiral cavities." Another notable advantage of this chiral capsule is that the resulting composites can be used in water without the need for organic solvents, which was a limitation of other designs.
Overall, this study has the potential to lead to affordable, high-performance chiroptically active composites, which in turn would pave the way to advances in cutting-edge optical technologies. "Our strategy allows for the straightforward introduction of chirality to various non-chiral dyes without elaborate synthetic modification, highlighting its potential application to polymer materials and catalysts," concludes Dr. Tanaka. "Based on the present findings, an ongoing research project in our laboratory focuses on multicomponent host−guest systems with higher chiroptical functions," he adds, hinting at future developments.
- Reference
| Authors: | Yoshihisa Hashimoto1, Yuya Tanaka1*, Daiya Suzuki2, Yoshitane Imai2, and Michito Yoshizawa1* |
|---|---|
| Title: | Chiroptically Active Host–Guest Composites Using a Terpene-Based Micellar Capsule |
| Journal: | Journal of the American Chemical Society |
| Affiliations: |
1Laboratory for Chemistry and Life Science, Institute of Integrated Research, Institute of Science Tokyo,
Japan 2Graduate School of Science and Engineering, Kindai University, Japan |
| DOI: |
10.1021/jacs.4c07193 |
Related articles
- A First for Ferrocene: Organometallic Capsule with Unusual Charge-Transfer Interactions | Tokyo Tech
- A Nanocapsulation Strategy for Facile Analysis and Processing of Insoluble Aromatic Polymers in Water | Tokyo Tech
- Enabling Nanoscale Thermoelectrics with a Novel Organometallic Molecular Junction | Tokyo Tech
- Creating a nanospace like no other | Tokyo Tech
- Nanosized container with photoswitches: release of cargo upon irradiation in water | Tokyo Tech
- Biomimetics: Artificial receptor distinguishes between male and female hormones | Tokyo Tech
- All wired up: New molecular wires for single-molecule electronic devices | Tokyo Tech
- Sweet success: Nanocapsule perfectly binds sucrose in water | Tokyo Tech
- Michito Yoshizawa - Novel nanostructures using self-assembly | Tokyo Tech
- Yuya Tanaka | Researcher Finder - Science Tokyo STAR Search
- Michito Yoshizawa | Researcher Finder - Science Tokyo STAR Search
- Yoshizawa & Sawada Laboratory
- Institute of Integrated Research
- Chemical Science and Engineering Graduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Chemical Science and Engineering Undergraduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- School of Materials and Chemical Technology
- Kindai University
- Research
Further information
Institute of Integrated Research, Institute of Science Tokyo
Assistant Professor Yuya Tanaka
Email:ytanaka@res.titech.ac.jp
Institute of Integrated Research, Institute of Science Tokyo
Professor Michito Yoshizawa