Chemical Science and Engineering News
Transition-Metal-Free Zeolite Catalyst for Direct Conversion of Methane to Methanol
Researchers present aluminosilicate ferrierite zeolite catalyst as a promising material for carrying out the conversion reaction
Direct oxidation of methane to methanol is dominated by transition- or noble-metal-based catalysts, thus making the reaction quite expensive. To make the process efficient and cost-effective, researchers from Tokyo Tech developed a transition-metal-free aluminosilicate ferrierite zeolite catalyst that can produce methanol by using methane and nitrous oxide as starting materials. The new catalyst ensures excellent methanol production efficiency, one of the highest recorded rates in the literature thus far.
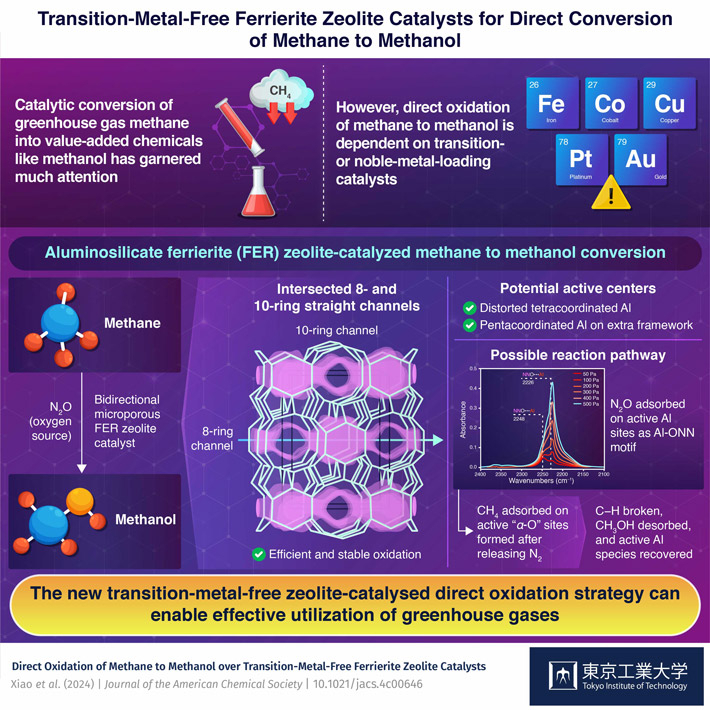
In light of the waste-to-wealth movement, technology for converting greenhouse gases into value-added materials has gained significant attention in recent years. One such technology is the catalytic conversion of methane into methanol, a widely used industrial solvent and raw material for chemical synthesis. The industrial process for conversion of methane to methanol is extremely energy and resource-intensive. In the past decade, scientists have developed several catalyst systems that can enable direct oxidation of methane to methanol. However, most of them are based on rare and expensive transition or noble metals.
In a recent study, a group of researchers, led by Associate Professor Toshiyuki Yokoi and including Assistant Professor Peipei Xiao, both from the Nanospace Catalysis Unit of the Institute of Innovative Research at Tokyo Institute of Technology, Japan, found a solution to the existing problem in the form of transition-metal-free aluminosilicate ferrierite (FER) zeolite. FER is a 2-dimensional zeolite with 8-ring channels and intersected 10-ring channels known to be highly stable towards chemical and thermal treatments. Taking advantage of this unique structural property, Yokoi and his team demonstrated for the first time the direct oxidation of methane to methanol using nitrous oxide as the source of oxygen. The findings of this study were made available online on April 1, 2024, and published in the Journal of the American Chemical Society![]() on April 10, 2024.
on April 10, 2024.
"Transition-metal exchanged zeolites have widely been used in gas- and liquid-phase reaction systems. Such moieties have been reported to have active sites inspired by natural biocatalytic enzyme systems. While the catalysts with transition-metal loading are quite popular, zeolite catalysts consisting of only main group elements for direct oxidation of methane to methanol remained unexplored," remarks Yokoi when asked about the motivation behind developing FER zeolites.
To get a clear picture of the active sites in the zeolite catalyst, the team conducted Fourier transform infrared spectroscopy (FTIR) and magnetic resonance spectroscopy. The tests revealed distorted tetracoordinated Al in the framework and pentacoordinated Al in the extra framework formed during the activation, calcination, and reaction processes. These structures were established as potential active centers. The team also probed the possible reaction pathway with the help of nitrous oxide adsorption FTIR spectra and observation of reaction performances under different oxidants. They found that the process began with the absorption of nitrous oxide on the active Al sites and the formation of "α-O" leading to the release of nitrogen. This was followed by the adsorption of methane on the active "α-O" sites and the breaking of C−H bonds. After the bond breaking, methanol was obtained via desorption followed by recovery of the active Al species.
The new transition-metal-free zeolite-catalyzed direct oxidation process enabled 305 μmol g−1 min−1 methanol production rate with 89% methanol and 10% dimethyl ether selectivity, thus, recording one of the highest performances reported in the literature, even better than many transition metal-loaded catalysts.
"Our findings will open up a brand-new avenue for the development of direct oxidation of methane to methanol on transition-metal-free aluminosilicate zeolites, thus enabling the utilization of methane and nitrous oxide to other useful chemicals on aluminosilicate zeolites with different topological structures," adds Yokoi, describing the real-life implications of their research.
Overall, the new strategy proposed in this study can create a positive environmental impact by reducing the amount of greenhouse gases circulating in the atmosphere and adding to the economy by facilitating the synthesis of valuable chemicals from unwanted raw materials.
- Reference
| Authors : | Peipei Xiao1, Yong Wang1, Yao Lu1, Kengo Nakamura1, Nobuki Ozawa2,3, Momoji Kubo2,3, Hermann Gies1,4, and Toshiyuki Yokoi1,5,* |
|---|---|
| Title : | Direct Oxidation of Methane to Methanol over Transition-Metal-Free Ferrierite Zeolite Catalysts |
| Journal : | Journal of the American Chemical Society |
| DOI : | 10.1021/jacs.4c00646 |
| Affiliations : | 1Nanospace Catalysis Unit, Institute of Innovative Research, Tokyo Institute of Technology, Japan 2New Industry Creation Hatchery Center, Institute for Materials Research, Tohoku University, Japan 3Institute for Materials Research, Tohoku University, Japan 4Institute of Geology, Mineralogy und Geophysics, Ruhr-University Bochum, Germany 5iPEACE223 Inc., Japan |
|
* Corresponding author's email: yokoi@cat.res.titech.ac.jp |
|
- A Novel Multifunctional Catalyst Turns Methane into Valuable Hydrocarbons | Tokyo Tech News
- Efficient Carbon Dioxide Reduction under Visible Light with a Novel, Inexpensive Catalyst | Tokyo Tech News
- New Photocatalyst Boosts Water Splitting Efficiency for Clean Hydrogen Production | Tokyo Tech News
- Lights, Catalyst, Reaction! Converting CO2 to Formic Acid Using an Alumina-supported, Iron-based Compound | Tokyo Tech News
- Integrating nanomaterial with light-absorbing molecule powers hydrogen production from water and sunlight | Tokyo Tech News
- FY2017 STAR grant recipients selected | Tokyo Tech News
- Toshiyuki Yokoi | Researcher Finder - Tokyo Tech STAR Search
- Nanospace Catalysis Unit
- Toshiyuki Yokoi Laboratory | School of Materials and Chemical Technology Laboratory Search Site
- Laboratory for Chemistry and Life Science, Institute of Innovative Research
- Institute of Innovative Research (IIR)
- Chemical Science and Engineering Graduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Chemical Science and Engineering Undergraduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- International Research Frontiers Initiative
- Tohoku University
- Latest Research News
Further Information
Associate Professor Toshiyuki Yokoi
Institute of Innovative Research, Tokyo Institute of Technology