Chemical Science and Engineering News
Alkyl-Aromatic Hybrid Micelles Formed from Emergent Umbrella-Shaped Molecules
Micelles are among the most widely employed molecular assemblies, with their features being governed by the subunit molecules, composed of hydrophobic and hydrophilic frameworks. So far, the design principle for the hydrophobic part of the subunits has been mostly focused on either linear alkyl chains or bent aromatic units. Now, a team at Tokyo Institute of Technology (Tokyo Tech) has developed unique alkyl-aromatic hybrid micelles displaying significantly higher performance in terms of assembly stability as well as guest binding, compared to previous alkyl micelles as well as aromatic micelles. Importantly, the uptake of large cyclic molecules yielded interpenetrated ring-structures with strong green emission in water.
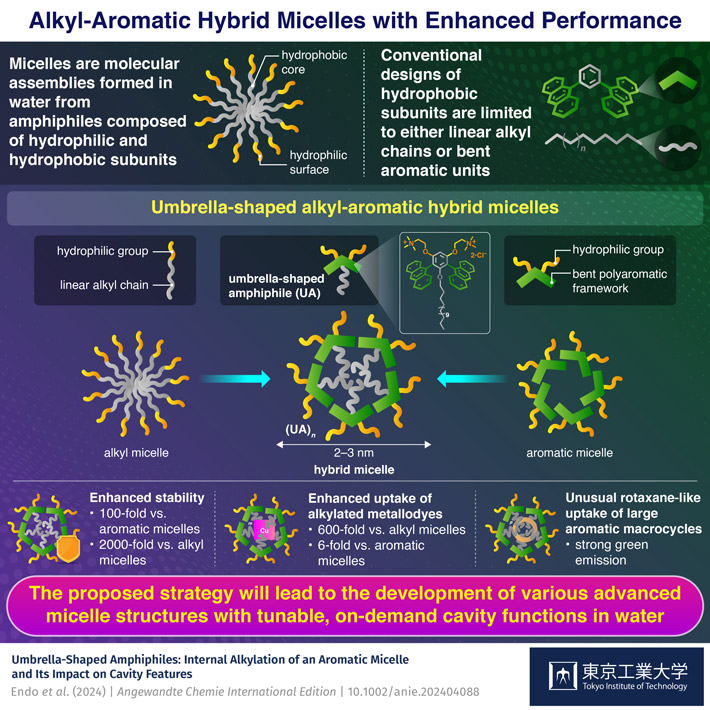
Micelles assemble in water from amphiphilic molecules, composed of hydrophilic and hydrophobic frameworks. They can be found all around us, for example in soaps, detergents, and shampoos. Their main application is the water-solubilization of insoluble molecules through encapsulation into hydrophobic cavities. These cavities are conventionally composed of linear alkyl-chains, providing good interactions with alkyl-based guests, yet poor interactions with aromatic compounds. In addition, the rather weak intermolecular alkyl-alkyl type interactions result in an overall poor stability against heat and dilution.
In 2013, Kei Kondo and Michito Yoshizawa from Tokyo Tech's Laboratory for Chemistry and Life Science in Japan reported a new class of micelles, so called aromatic micelles, formed from bent anthracene-based amphiphiles. Aromatic micelles show high stability in water and excellent host functions towards aromatic guests, however only moderately interact with alkylated frameworks. To develop new micelles with unprecedented properties, the group around Masaya Endo, Dr. Lorenzo Catti, and Prof. Yoshizawa now achieved the preparation of alkyl-aromatic hybrid micelles obtained from umbrella-shaped amphiphiles UA, published in Angewandte Chemie on April 15. The new amphiphiles feature a linear alkyl-chain flanked by two aromatic panels (i.e., anthracene units), and quantitatively self-assemble in water into small spherical micelles of ~2-3 nm in diameter, providing an alkyl core surrounded by an aromatic shell. The micellar alkyl cores are found to be highly compacted and shielded from water as indicated by solvent-repsonsive fluorescent guest probes.
Interestingly, the hexadecyl-based hybrid micelle (UA16)n showed excellent stability against dilution, with an approximately 100-fold and 2000-fold increase in stability as compared to aromatic and alkyl micelles, respectively. The high stability is believed to arise from combined alkyl-alkyl, aromatic-aromatic, and alkyl-aromatic interactions. Micelle (UA16)n furthermore resisted disassembly up to 120 °C, making it suitable for applications under extreme conditions.
"Besides very high stability, the new hybrid micelles provide superior uptake functions toward large alkylated metallodyes. Control experiments with alkyl and aromatic micelles revealed an approximately 600-fold and 6-fold enhancement in uptake efficiency, respectively. Simple mixing of alkyl and aromatic micelles was found to provide no significant enhancement, emphasizing the importance of the new hybrid design", says Dr. Catti.
Finally, the group revealed the efficient uptake/water-solubilization of large aromatic macrocycles, i.e., cycloparaphenylenes (CPPs), provided by Dr. Tsuchido from Tokyo University of Science. These macrocycles are composed of covalently linked benzene rings and feature intruiging physicochemical properties. For example, subjecting UA16 and 9CPP, constructed from nine linked benzene rings, to a grinding protocol yielded a clear solution containing micellar composites encapsulating in average a single molecule of 9CPP. A combination of experimental and computational data revealed that the alkyl chains of UA16 interpenetrate the 9CPP ring structures, providing so-called pseudorotaxane structures, rarely reported for unfunctionalized cycloparaphenylenes. Additionally, the 9CPP based composites showed strong green emission in water, with a quantum yield of 35%. Larger CPPs were likewise encapsulated with high efficiency.
The team concludes: "While the focus of this study was placed on the combination of alkyl and aromatic hydrophobic frameworks, we are convinced that the generality of the method will allow us to develop a wide range of powerful new amphiphiles by exploring different combinations, for example ones including peptide or fluoroalkane frameworks. The corresponding hybrid micelles are expected to find use as on-demand host structures for solubilization, stabilization, and targeted delivery applications."
- Reference
| Authors : | Masaya Endo1, Shinji Aoyama1, Yoshitaka Tsuchido2, Lorenzo Catti1* and Michito Yoshizawa1* |
|---|---|
| Title : | Umbrella-Shaped Amphiphiles: Internal Alkylation of an Aromatic Micelle and Its Impact on Cavity Features |
| Journal : | Angewandte Chemie International Edition |
| DOI : | 10.1002/anie.202404088 |
| Affiliations : |
1 Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology 2 Department of Chemistry, Faculty of Science, Tokyo University of Science |
- A First for Ferrocene: Organometallic Capsule with Unusual Charge-Transfer Interactions | Tokyo Tech News
- A Nanocapsulation Strategy for Facile Analysis and Processing of Insoluble Aromatic Polymers in Water | Tokyo Tech News
- Just Mix It Up: New Synthetic Method for Making Amphiphilic Molecules without Additives | Tokyo Tech News
- Nanosized container with photoswitches: release of cargo upon irradiation in water | Tokyo Tech News
- Biomimetics: Artificial receptor distinguishes between male and female hormones | Tokyo Tech News
- Sweet success: Nanocapsule perfectly binds sucrose in water | Tokyo Tech News
- FY2014 STAR Grant Recipients Selected | Tokyo Tech News
- Michito Yoshizawa - Novel nanostructures using self-assembly | Research Stories | Research
- Lorenzo Catti | Researcher Finder - Tokyo Tech STAR Search
- Michito Yoshizawa | Researcher Finder - Tokyo Tech STAR Search
- Yoshizawa and Sawada Laboratory
- Laboratory for Chemistry and Life Science, Institute of Innovative Research
- Institute of Innovative Research (IIR)
- Chemical Science and Engineering Graduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Chemical Science and Engineering Undergraduate Major|Education|Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Latest Research News
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Assistant Professor Lorenzo Catti
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology
Email catti.l.aa@m.titech.ac.jp
Professor Michito Yoshizawa
Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology