Chemical Science and Engineering News
Breakthrough in blending metals: Precise control of multimetallic one-nanometer cluster formation achieved
Researchers in Japan have found a way to create innovative materials by blending metals with precision control. Their approach, based on a concept called atom hybridization1, opens up an unexplored area of chemistry that could lead to the development of advanced functional materials.
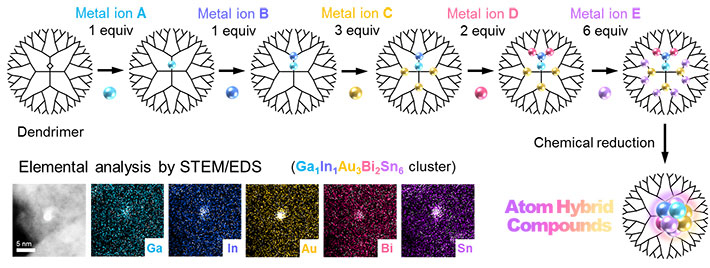
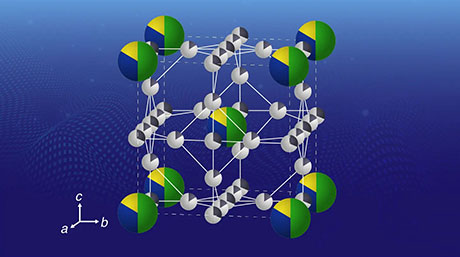
Figure 1. A conceptual image of the atom hybridization method
Five metal elements are blended here in a small cluster on a one-nanometer scale.
Background
Multimetallic clusters — typically composed of three or more metals — are garnering attention as they exhibit properties that cannot be attained by single-metal materials. If a variety of metal elements are freely blended, it is expected that as-yet-unknown substances are discovered and highly-functional materials are developed. So far, no one had reported the multimetallic clusters blended with more than four metal elements so far because of unfavorable separation of different metals. One idea to overcome this difficulty is miniaturization of cluster sizes to one-nanometer scale, which forces the different metals to be blended in a small space. However, there was no way to realize this idea.
Overview
A team, including Takamasa Tsukamoto, Takane Imaoka, Kimihisa Yamamoto and colleagues, has developed an atom hybridization method, which has realized the first-ever synthesis of multimetallic clusters consisting of more than five metal elements with precise control of size and composition. This method employs a dendrimer template2 that serves as a tiny “scaffold” to enable controlled accumulation of metal salts. After precise uptake of the different metals into the dendrimer, multimetallic clusters are obtained by chemical reduction. (See Figure 1.) In contrast, a conventional method without the dendrimer yields enlargement of cluster sizes and separation of different metals. (See Figure 2.)

Figure 2. A conventional method without the dendrimer template
Large clusters (of over 10-nanometers) are obtained, and different metals are separated from each other.
The team successfully demonstrated the formation of five-element clusters composed of gallium (Ga), indium (In), gold (Au), bismuth (Bi) and tin (Sn), as well as iron (Fe), palladium (Pd), rhodium (Rh), antimony (Sb) and copper (Cu), and a six-element cluster consisting of Ga, In, Au, Bi, Sn and platinum (Pt). Additionally, they hint at the possibility of making clusters composed of eight metals or more.
Future Development
This atom hybridization method using the dendrimer template can synthesize ultrasmall multimetallic clusters with precise control of size and composition. There are more than 90 metals in the periodic table. With infinite combinations of metal elements, atomicity and composition, this method will open up a new field in chemistry on a one-nanometer scale. The current study marks a major step forward in creating such as-yet-unknown innovative materials.
Technical terms
A method for synthesis of multimetallic clusters on a one-nanometer scale by using a dendrimer2 as a nanosized template. Various metal ions can be taken up into the dendrimer structure with various combinations. Multimetallic clusters are obtained by chemical reduction of these metal ions on the template.
A dendrimer is a specific macromolecule having repetitively branched structures. The team utilizes a dendrimer template suitably designed for the assembly of metal atoms in controlling the number of atoms.
Reference
| Authors : | Takamasa Tsukamoto1, Tetsuya Kambe1, 2, Aiko Nakao3, Takane Imaoka1, 2, 4, Kimihisa Yamamoto1, 2,* |
|---|---|
| Title of original paper : | Atom-hybridization for synthesis of polymetallic clusters |
| Journal : | Nature Communications |
| DOI : | 10.1038/s41467-018-06422-8 |
| Affiliations : |
1 JST-ERATO, Yamamoto Atom Hybrid Project, Institute of Innovative Research, Tokyo Institute of Technology 2 Laboratory for Chemistry and Life Science, Tokyo Institute of Technology 3 RIKEN 4 JST-PRESTO |
- The Exploratory Research for Advanced Technology (ERATO)
- How a tetrahedral substance can be more symmetrical than a spherical atom |Tokyo Tech News
- A Preparative-Scale Reaction Using Platinum Clusters with a Single-Digit Atomicity Realized | Tokyo Tech News
- One-Nanometer Trimetallic Alloy Particles Created | Tokyo Tech News
- New aspect of atom mimicry for nanotechnology applications | Tokyo Tech News
- Controllable light-emitting materials to advance light sensing and nano-medicine | Tokyo Tech News
- A step forward for fuel cell technology | Tokyo Tech News
- Yamamoto-Imaoka Laboratory
- Takamasa Tsukamoto Website
- Researcher Profile | Tokyo Tech STAR Search - Takamasa Tsukamoto
- Researcher Profile | Tokyo Tech STAR Search - Kimihisa Yamamoto
- Researcher Profile | Tokyo Tech STAR Search - Tetsuya Kambe
- Researcher Profile | Tokyo Tech STAR Search - Takane Imaoka
- Hybrid Materials Unit, Institute of Innovative Research (IIR)
- Laboratory for Chemistry and Life Science, Institute of Innovative Research
- Latest Research News
Further Information
Professor Kimihisa Yamamoto
Institute of Innovative Research,
Tokyo Institute of Technology
Email yamamoto@res.titech.ac.jp
Tel +81-45-924-5260
Contact
Public Relations Section,
Tokyo Institute of Technology
Email media@jim.titech.ac.jp
Tel +81-3-5734-2975