Life Science and Technology News
【Labs spotlight】Kuroda Laboratory
The Department has a variety of laboratories for Life Science and Technology, in which cutting-edge innovative research is being undertaken not only in basic science and engineering but also in the areas of medicine, pharmacy, agriculture, and multidisciplinary sciences.
This "Spotlight" series features a laboratory from the Department and introduces you to the laboratory's research projects and outcomes. This time we focus on Kuroda Laboratory.

Areas of Supervision
Primary/Science and Technology for health Care and Medicine
Secondary/Life Science and Technology, Human Centered Science and Biomedical Engineering
Professor Kumi Kuroda![]()
| Office | Room 409, B1 building, Suzukakedai campus |
|---|---|
| Keywords | Behavioral Neuroscience, Parental behavior, Attachment, Affiliative social behavior, Hypothalamus, Preoptic area |
| WEBsite URL | Kuroda Laboratory |
Research interest
Neural Mechanisms in parent-infant relationships and affiliative sociality
As social beings, humans rely on intimate interactions within families, schools, and workplaces, which are essential for a fulfilling life. Such human sociality has its basis in innate social behaviors conserved in mammals. In particular, parental care is one of the most basic social behaviors among animals and forms the basis of complex affiliative sociality including cooperation, altruism, and empathy. We aim to scientifically understand the neural mechanisms of parent-offspring relationships to support the well-being of families and communities.
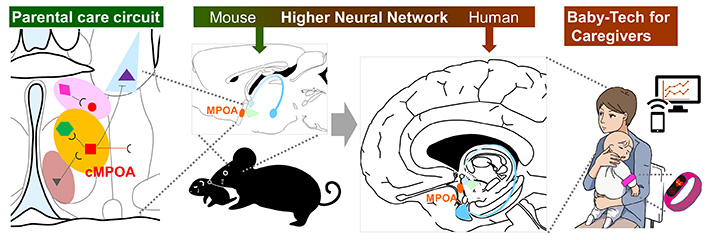
Neural Circuit Mechanisms of Mammalian Parenting Behavior
Mammalian infants are born immature and require extensive care for survival. Parents, especially mothers, attend, feed, and protect their offspring. Mammals possess brain elements essential for parenting , which are shaped into functional neural circuits through early-life nurturing experiences, social interactions, and practices as new parents.
The brain hub for parental behaviors is the medial preoptic area (MPOA in the above figure) in the basal forebrain, located anterior to the hypothalamus. In our 2013 and 2015 reports, we found that within the MPOA, the central part of the cMPOA is indispensable for mouse parental behaviors by mothers, fathers, and alloparents. As the molecularly-defined neural circuit selectively regulates parental care, we next identified Calcr (calcitonin receptor)-expressing neurons in this cMPOA in 2021![]() (see the link for details). Expression of Calcr increases dramatically in the cMPOA and facilitates the nurturing motivation of maternal mice even under risky conditions. The same neuromolecular circuit is responsible for infant care in a family-living primate species common marmosets (2022
(see the link for details). Expression of Calcr increases dramatically in the cMPOA and facilitates the nurturing motivation of maternal mice even under risky conditions. The same neuromolecular circuit is responsible for infant care in a family-living primate species common marmosets (2022![]() , 2023), suggesting that this evolutionary conserved neural mechanism should be involved in parental care in humans.
, 2023), suggesting that this evolutionary conserved neural mechanism should be involved in parental care in humans.
Affiliative sociality evolved from parenting
Generally, female mammals exhibit more sociable behavior than their male counterparts. In diurnal primates, females tend to live in groups, while males may not. Such female-biased group living is supposed to be evolved for the benefit of parental care.
Our research in 2022![]() indicated that the MPOA parenting circuit (Calcr and its peptide ligand Amylin) is also involved in affiliative sociality in group-living female mice. MPOA Amylin expression is maintained by free social interaction and facilitates contact-seeking with peer mice. Amylin-Calcr signaling may mediate mutual facilitation of group living and maternal care in the cMPOA.
indicated that the MPOA parenting circuit (Calcr and its peptide ligand Amylin) is also involved in affiliative sociality in group-living female mice. MPOA Amylin expression is maintained by free social interaction and facilitates contact-seeking with peer mice. Amylin-Calcr signaling may mediate mutual facilitation of group living and maternal care in the cMPOA.
A large body of evidence has suggested that empathy and altruism have first evolved for parental care in mammals. Studying the neural mechanisms of parental care should also contribute to a better understanding of sociality in general.
Infant attachment to their primary caregivers
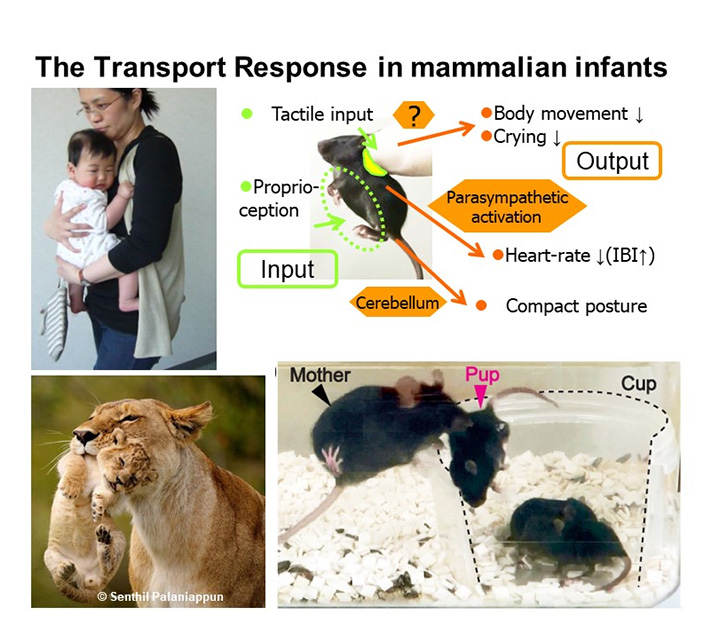
Infants and children act in forming and maintaining the bond with parents (or primary caregivers), by approaching and signaling toward caregivers. These behaviors are collectively called attachment behaviors. The brain areas and neural circuits responsible for various attachment behaviors are still largely unknown. In 2013![]() , we presented the first scientific evidence that infants cry less and become docile as soon as their parents carry them in both mice and human infants (video link
, we presented the first scientific evidence that infants cry less and become docile as soon as their parents carry them in both mice and human infants (video link![]() ), the phenomenon termed the Transport Response.
), the phenomenon termed the Transport Response.
We then reported in 2022![]() on how this transport response can be used to calm excessive crying and to promote sleep (see video at top of page). Specifically, they found that holding the baby snugly against the body and walking for five consecutive minutes while trying not to stop too often significantly reduced crying and nearly half of the babies fell asleep.
on how this transport response can be used to calm excessive crying and to promote sleep (see video at top of page). Specifically, they found that holding the baby snugly against the body and walking for five consecutive minutes while trying not to stop too often significantly reduced crying and nearly half of the babies fell asleep.
Our goal is to leverage these findings in developing wearable devices and applications to assist childcare, potentially preventing postpartum depression and child abuse triggered by excessive crying.
Selected publications
Kurachi T, Shinozuka K, Yoshihara C, Yano-Nashimoto S, Murayama AY, Hata J, Haga, Y, Okano H, Kuroda KO, “Distinct roles of amylin and oxytocin signaling in intrafamilial social behaviors at the medial preoptic area of common marmosets”, Communications Biology, in press. (2023)
Kazutaka Shinozuka, Saori Yano-Nashimoto, Chihiro Yoshihara, Kenichi Tokita, Takuma Kurachi, Ryosuke Matsui, Dai Watanabe, Ken-ichi Inoue, Masahiko Takada, Keiko Moriya-Ito, Hironobu Tokuno, Michael Numan, Atsuko Saito, Kumi O. Kuroda, "A calcitonin receptor-expressing subregion of Masahiko Takada, Keiko Moriya-Ito, Hironobu Tokuno, Michael Numan, Atsuko Saito, Kumi O. Kuroda, "A calcitonin receptor-expressing subregion of the medial preoptic area is involved in alloparental tolerance in common marmosets", Communications Biology, 5, Article number: 1243 (2022) Website![]()
Ohmura N, Okuma L, Truzzi A, Shinozuka A, Saito A, Yokota S, Bizzego A, Miyazawa E, Shimizu M, Esposito G, Kuroda KO: "A method to soothe and promote sleep in crying infants utilizing the Transport Response", Current Biology, 32(20), 4521-4529.e4 (2022) Website![]()
Fukumitsu K, Kaneko M, Maruyama T, Yoshihara C, Huang AJ, McHugh TJ, Itohara S, Tanaka M, Kuroda KO: "Amylin-Calcitonin receptor signaling in the medial preoptic area mediates affiliative social behaviors in female mice", Nature Communications, 13, Article number: 709 (2022) PDF![]()
Yoshihara C, Tokita K, Maruyama T, Kaneko M, Tsuneoka Y, Fukumitsu K, Miyazawa E, Shinozuka K, Huang AJ, Nishimori K, McHugh TJ, Tanaka M, Itohara S, Itohara S, "Calcitonin receptor signaling in the medial preoptic area enables risk-taking maternal care", Cell Reports, 35(9), Kuroda KO Touhara K, Miyamichi K, Kuroda KO: "Calcitonin receptor signaling in the medial preoptic area enables risk-taking maternal care", Cell Reports, 35(9), 109204 (2021) Website![]()
Kimi Kuroda (ed.), Child Abuse Prevention and Caregiver Support, Iwasaki Academic Press, 2022 Website![]()
Kimi Kuroda, "The Brain that Creates Parent-Child Connections," Connecting Brain Science 2016, 281-313 Website![]()
- Original Papers
https://researchmap.jp/oyako/published_papers![]()
- book
https://kurodalab.net/page/publication/book![]()
- Reviews in Japanese
https://kurodalab.net/page/publication/japanese-review![]()
Contact
Professor Kumi Kuroda
Room 409, B1 building, Suzukakedai campus
E-mail : kurodalab[at]bio.titech.ac.jp
Tel / Fax : +8145-924-5441
*Find more about the lab and the latest activities at the lab site![]() .
.
*May 1, 2025:Some of the content has been updated with the latest information.