Life Science and Technology News
【Labs spotlight】 Koshikawa Laboratory

Areas of Supervision
Primary/Science and Technology for health Care and Medicine
Secondary/Life Science and Technology, Human Centered Science and Biomedical Engineering
Professor Naohiko Koshikawa![]()
| Degree | PhD 1995, Yokohama City University |
|---|---|
| Areas of Research | Cancer Biology, Clinical Proteomics, Diagnostics and Therapeutics |
| Keywords | Extracellular Matrix, Metalloproteinase, Cancer, diagnosis, Biomarkers |
| Website | Koshikawa Laboratory |
Research interest
To understand the mechanisms of malignant progression of cancer cells induced by membrane-type matrix metalloproteinases (MT-MMP), we have consistently focused on membrane-type 1 matrix metalloproteinase (MT1-MMP) proteolysis using different technologies. Our goal is to develop innovative diagnostics and therapeutics targeting MT1-MMP and its related molecules.
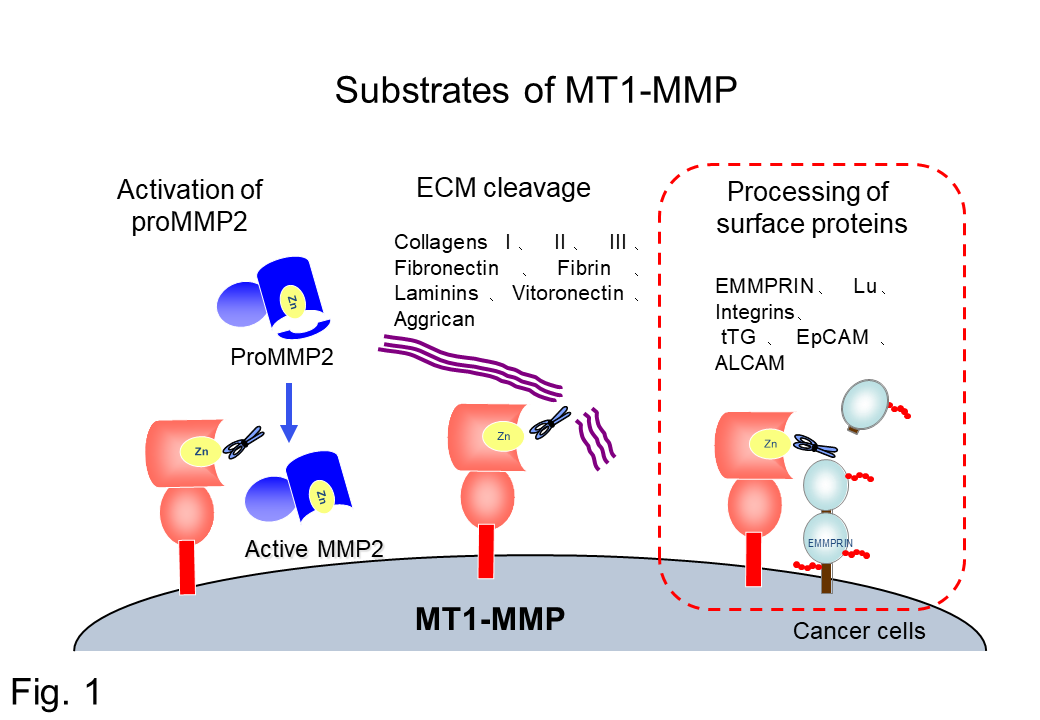
MT1-MMP was identified as a membrane-type metalloprotease in 1994, in Japan. Since MT1-MMP is highly expressed in invasive cancers, and can degrade collagen type IV in basement membranes by activating the MMP2 precursor for basement membrane disruption by invading cancer cells, MT1-MMP was believed to be a driving force for cancer metastases and invasion (Fig. 1).
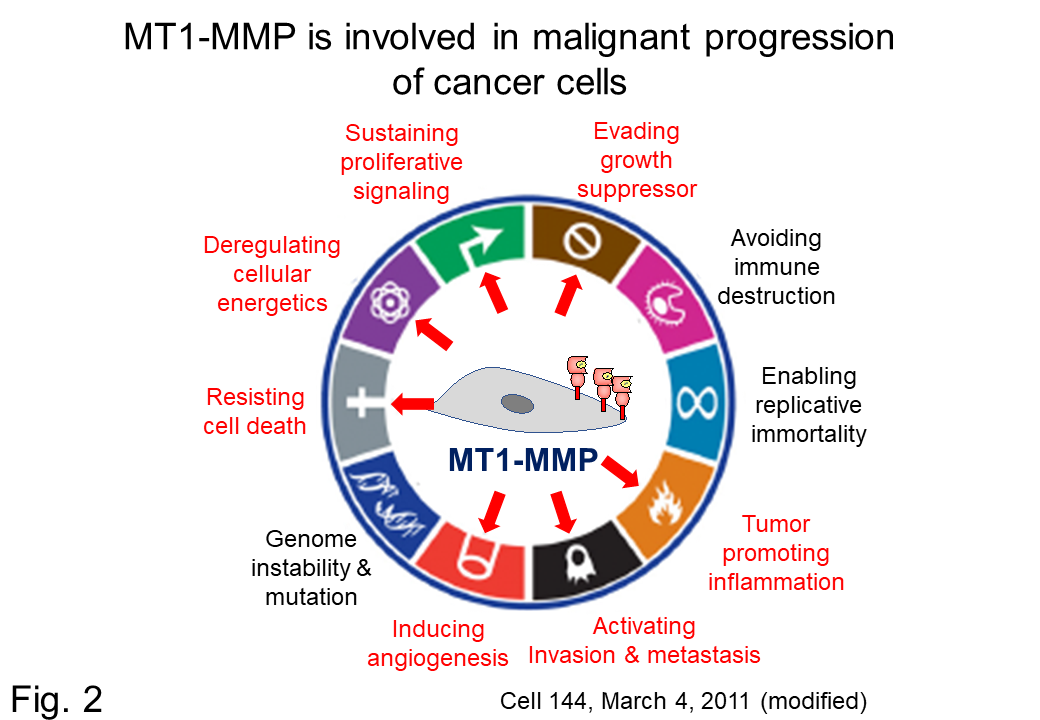
Recently, MT1-MMP has been shown to be involved in not only cancer metastases and invasion but also the malignant progression of cancer cells (Fig. 2). Therefore, understanding the molecular mechanisms of cancer cell malignant progression regulated by MT1-MMP can lead to the development of new cancer therapies, diagnostics, and drugs. Thus, we are focusing on a third MT1-MMP substrate and systematically investigating the possibility that partial cleavage (processing) of the substrate is involved in regulating new functions of membrane proteins (Fig. 2).
We have found that MT1-MMP at the cancer cell membrane i) interacts with a membrane-type growth factor and the tyrosine kinase receptor, which play critical roles in the transition of malignant signaling in cancer cells (Cancer Sci 2008); ii) enhances cancer malignant potential by processing and regulating the activity of these membrane proteins (Cancer Res 2010, 2015); and iii) produces a cancer-specific fragment(s) which is a specific biomarker for cancer diagnosis or a therapeutic target (Cancer Sci 2011, Cell Death & Diseases 2017).
These results strongly suggest that MT1-MMP, which is a powerful driving force of invasion and metastasis, contributes to the malignant progression of cancer cells by regulating the function of membrane proteins, and provides a new target for cancer therapy and diagnostics.
At present, we are advancing translational research based on the findings of basic research to date, and we will disseminate our original cancer treatment and diagnostics to society by determining the clinical usefulness (Proof of Concept: POCs) of seeds targeting MT1-MMP and related molecules.
Research findings
Selected publications
- 1.Nagano M, Hoshino D, Toshima J, Seiki M, Koshikawa N. NH2-terminal fragment of ZF21 protein suppresses tumor invasion via inhibiting the interaction of ZF21 with FAK: Cancer Science, in press.
- 2.Suzuki M, Muroi A, Nojima M, Numata A, Takasaki H, Sakai R, Yokose T, Miyagi Y, Koshikawa N. Utility of a Reverse Phase Protein Array to Evaluate Multiple Biomarkers in Diffuse Large B-Cell Lymphoma: Proteomics Clin Appl. 2020 Jan;14(1):e1900091. doi: 10.1002/prca.201900091.
- 3.Yasuda H, Nakagawa M, Kiyokawa H, Yoshida E, Yoshimura T, Koshikawa N, Itoh F, Seiki M. Unique Biological Activity and Potential Role of Monomeric Laminin-γ2 as a Novel Biomarker for Hepatocellular Carcinoma: A Review. Int J Mol Sci. 2019 Jan 8;20(1):226. doi: 10.3390/ijms20010226.
- 4.Takahashi Y, Hamasaki M, Aoki M, Koga K, Koshikawa N, Miyamoto S, Nabeshima K. Activated EphA2 Processing by MT1-MMP Is Involved in Malignant Transformation of Ovarian Tumours In Vivo. Anticancer Res. 2018 Jul;38(7):4257-4266. doi: 10.21873/anticanres.
- 5.Kikuchi K, Kozuka-Hata H, Oyama M, Seiki M, Koshikawa N. Identification of Proteolytic Cleavage Sites of EphA2 by Membrane Type 1 Matrix Metalloproteinase on the Surface of Cancer Cells. Methods Mol Biol. 2018;1731:29-37. doi:10.1007/978-1-4939-7595-2_3.
- 6.Koshikawa N, Minegishi T, Kiyokawa H, Seiki M. Specific detection of soluble EphA2 fragments in blood as a new biomarker for pancreatic cancer. Cell Death Dis. 2017 Oct 26;8(10):e3134. doi: 10.1038/cddis.2017.545.
- 7.Nakagawa M, Karashima T, Kamada M, Yoshida E, Yoshimura T, Nojima M, Inoue K, Shuin T, Seiki M, Koshikawa N. Development of a fully automated chemiluminescence immunoassay for urine monomeric laminin-γ2 as a promising diagnostic tool of non-muscle invasive bladder cancer. Biomarker Res. 2017 Oct 13;5:29. doi: 10.1186/s40364-017-0109-4.
- 8.Suzuki T, Minerva D, Nishiyama K, Koshikawa N Chaplain MAJ. Study on the tumor-induced angiogenesis using mathematical models. Cancer Sci. 2018 Jan;109(1):15-23. doi: 10.1111/cas.13395.
- 9.Kiyokawa H, Yasuda H, Oikawa R, Okuse C, Matsumoto N, Ikeda H, Watanabe T, Yamamoto H, Itoh F, Otsubo T, Yoshimura T, Yoshida E, Nakagawa M, Koshikawa N, Seiki M. Serum monomeric laminin-γ2 as a novel biomarker for hepatocellular carcinoma. Cancer Sci. 2017 Jul;108(7):1432-1439. doi: 10.1111/cas.13261.
- 10.Tatsukawa R, Koga K, Aoki M, Koshikawa N, Imafuku S, Nakayama J, Nabeshima K. Immunohistochemical demonstration of EphA2 processing by MT1-MMP in invasive cutaneous squamous cell carcinoma. Virchows Arch. 2016 Jul;469(1):25-34. doi: 10.1007/s00428-016-1934-9.
- 11.Kamada M, Koshikawa N, Minegishi T, Kawada C, Karashima T, Shuin T, Seiki M. Urinary laminin-γ2 is a novel biomarker of non-muscle invasive urothelial carcinoma. Cancer Sci. 2015 Dec;106(12):1730-7. doi: 10.1111/cas.12832.
- 12.Koshikawa N, Hoshino D, Taniguchi H, Minegishi T, Tomari T, Nam SO, Aoki M, Sueta T, Nakagawa T, Miyamoto S, Nabeshima K, Weaver AM, Seiki M. Proteolysis of EphA2 Converts It from a Tumor Suppressor to an Oncoprotein. Cancer Res. 2015 Aug 15;75(16):3327-39. doi: 10.1158/0008-5472.CAN-14-2798.
- 13.Kikuchi K, Noguchi A, Kasajima R, Miyagi Y, Hoshino D, Koshikawa N, Kubota A, Yokose T, Takano Y. Association of SIRT1 and tumor suppressor gene TAp63 expression in head and neck squamous cell carcinoma. Tumour Biol. 2015 Sep;36(10):7865-72. doi: 10.1007/s13277-015-3515-y.
- 14.Ito-Kureha T, Koshikawa N, Yamamoto M, Semba K, Yamaguchi N, Yamamoto T, Seiki M, Inoue J. Tropomodulin 1 expression driven by NF-κB enhances breast cancer growth. Cancer Res. 2015 Jan 1;75(1):62-72. doi: 10.1158/0008-5472.
- 15.Koshikawa N, Mizushima H, Minegishi T, Eguchi F, Yotsumoto F, Nabeshima K, Miyamoto S, Mekada E, Seiki M. Proteolytic activation of heparin-binding EGF-like growth factor by membrane-type matrix metalloproteinase-1 in ovarian carcinoma cells. Cancer Sci. 2011 Jan;102(1):111-6. doi:10.1111/j.1349-7006.
- 16.Koshikawa N, Mizushima H, Minegishi T, Iwamoto R, Mekada E, Seiki M. Membrane type 1-matrix metalloproteinase cleaves off the NH2-terminal portion of heparin-binding epidermal growth factor and converts it into a heparin-independent growth factor. Cancer Res. 2010 Jul 15;70(14):6093-103. doi:10.1158/0008-5472.CAN-10-0346.
- 17.Niiya D, Egawa N, Sakamoto T, Kikkawa Y, Shinkawa T, Isobe T, Koshikawa N, Seiki M. Identification and characterization of Lutheran blood group glycoprotein as a new substrate of membrane-type 1 matrix metalloproteinase 1 (MT1-MMP): a systemic whole cell analysis of MT1-MMP-associating proteins in A431 cells. J Biol Chem. 2009 Oct 2;284(40):27360-9. doi:10.1074/jbc.M109.029124.
- 18.Tomari T, Koshikawa N Uematsu T, Shinkawa T, Hoshino D, Egawa N, Isobe T,Seiki M. High throughput analysis of proteins associating with a proinvasive MT1-MMP in human malignant melanoma A375 cells. Cancer Sci. 2009 Jul;100(7):1284-90. doi: 10.1111/j.1349-7006.2009.01173.x.
- 19.Koshikawa N, Minegishi T, Nabeshima K, Seiki M. Development of a new tracking tool for the human monomeric laminin-gamma 2 chain in vitro and in vivo. Cancer Res. 2008 Jan 15;68(2):530-6. doi: 10.1158/0008-5472.CAN-07-5269.
- 20.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000 Feb 7;148(3):615-24. doi: 10.1083/jcb.148.3.615.
- 21.Koshikawa N, Moriyama K, Takamura H, Mizushima H, Nagashima Y, Yanoma S, Miyazaki K. Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999 Nov 1;59(21):5596-601. PMID: 10554040.
Contact
Professor Naohiko Koshikawa
Room 612, B1 building, Suzukakedai campus
E-mail : nkoshi@bio.titech.ac.jp
*Find more about the lab and the latest activities at the lab site![]() .
.
*May 1, 2025:Some of the content has been updated with the latest information.
*January 6, 2026:Some of the content has been updated with the latest information.