Life Science and Technology News
A reliable, easy-to-use mouse model for investigating bone metastasis
Researchers at Tokyo Tech propose an improved mouse model that could revolutionize bone metastasis research. Their method, which involves injecting cancer cells via the so-called caudal artery in the mouse tail, overcomes many limitations of traditional mouse models. The new model could thus open a new chapter in the development of therapeutic strategies for bone metastasis and cancer progression.
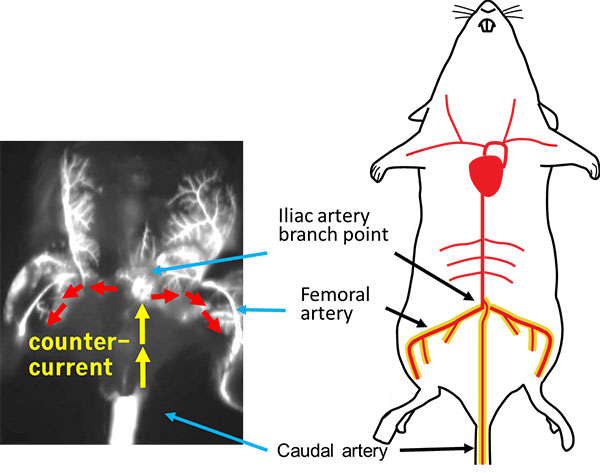
Figure 1. The caudal artery (CA) as a new route for injection
The study showed that cancer cells can be delivered in a direction counter to the blood flow in order to reach the branch point of the iliac artery, which sends blood to the lower limbs via the femoral arteries. The delivery route was confirmed using real-time near infrared fluorescent imaging.
In a study published in Nature Communications, a group of researchers led by Takahiro Kuchimaru* and Shinae Kondoh of Tokyo Tech present a mouse model that could greatly improve understanding of the underlying biology of bone metastasis.
It is widely known that metastasis — the spread of cancer cells from a primary tumor to other parts of the body — is one of the main causes of cancer mortality in humans. Bone metastasis commonly occurs when cancer cells spread to the bone from tumors originating, for example, in the prostate, breast, lung and kidney.
Experimental mouse models provide vital clues as to how cancer cells proliferate and how treatments could be developed. For the last 20 years, a model based on intracardiac (IC) injection has been considered the "gold standard" for inducing bone metastasis. This model involves injecting cancer cells directly into the left ventricle of the mouse heart. It requires a high degree of technical expertise, and even when performed successfully, the number of cancer cells that can be injected at any one time is limited. Another drawback is that the IC model tends to be more suitable for studying cancer cell lines that have a relatively high metastatic ability, ruling out analysis of "weaker" cancer cell lines.
In contrast, the new method developed by Kondoh's group involves injecting cancer cells via the caudal artery (CA) in the mouse tail — a procedure that can be performed much more easily as the artery is visible on the body surface. (See figures 1 and 2.) This method allows researchers to inject a larger number of cancer cells without causing acute death: In the present study, around one million cells were injected without any acute death. Moreover, the new method provides a new way of studying cancer cell lines with low bone metastatic potential.
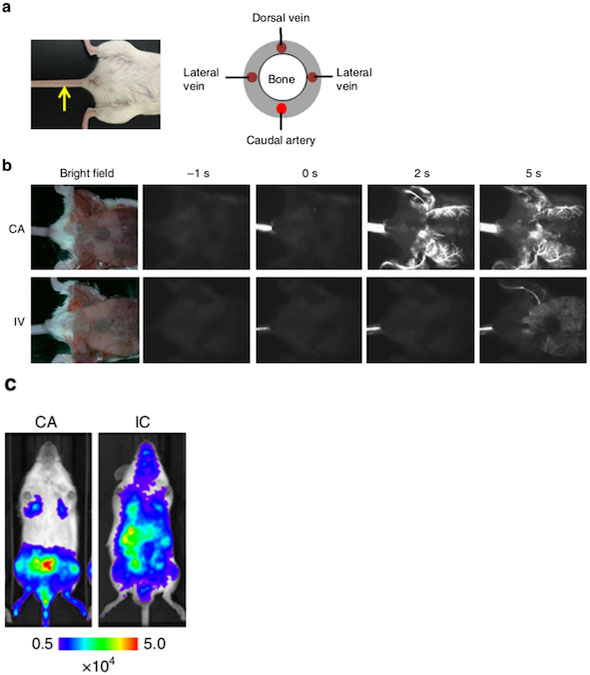
Figure 2. Caudal artery (CA) injection efficiently delivered cancer cells to the bone marrow of the hind limbs in mice.
a: Location of the caudal artery in a mouse (left) and a schematic cross-section of the mouse tail (right). The yellow arrow indicates the caudal artery along the tail.
b: Comparison of fluorescence images after CA or tail vein (IV) injection of near-infrared II nanoparticles.
c: Bioluminescence images at 30 minutes after injecting lung carcinoma cells expressing firefly luciferase (LLC/luc) via the CA (left) or left ventricle of the heart (right).
The researchers emphasize that the CA model predominantly ensures that bone metastasis develops in the hind limbs with much higher efficiency.
Using bioluminescence (BL) imaging, the team was able to detect bone metastasis just five to twelve days after CA injection of all cell lines examined.
"Overall, the results demonstrated that CA injection provides a reliable method to develop bone metastasis by increasing the delivery efficiency of a wide variety of cancer cell lines to the bone marrow of the hind limbs in mice," they say.
In addition, the CA model enables scientists to monitor the progression of bone metastasis over a longer period of time compared with the IC model due to reduced incidence of lethal metastasis in other organs. This represents a big step forward for investigating cancer cell dormancy and reactivation in greater depth.
The researchers conclude: "Our model may open a new avenue for understanding the bone metastatic processes and development of drugs preventing bone metastasis and recurrence."
* Takahiro Kuchimaru, the first author of the study, worked at the Kondoh Laboratory within the School of Life Science and Technology, Tokyo Institute of Technology, from 2012 to 2018. He is currently Assistant Professor at the Center for Molecular Medicine, Jichi Medical University.
Reference
| Authors : | Takahiro Kuchimaru, Naoya Kataoka, Kenji Nakagawa, Tatsuhiro Isozaki, Hitomi Miyabara, Misa Minegishi, Tetsuya Kadonosono, Shinae Kizaka-Kondoh |
|---|---|
| Title of original paper : | A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries |
| Journal : | Nature Communications |
| DOI : | 10.1038/s41467-018-05366-3 |
| Affiliations : | Department of Life Science and Technology, School of Life Science and Technology, Tokyo Institute of Technology |
- A novel non-invasive imaging probe for fast and sensitive detection of cancer | Tokyo Tech News
- Illuminating detection of deep cancers | Tokyo Tech News
- Shinae Kondoh - Optical imaging to detect cancer cells | Research Stories | Research
- Labs spotlight #28 - Kondoh Laboratory - | Life Science and Technology News
- Kondoh Lab
- Researcher Profile | Tokyo Tech STAR Search - Shinae Kondoh
- Department of Life Science and Technology, School of Life Science and Technology
- Latest Research News
Further Information
Professor Shinae Kondoh
School of Life Science and Technology,
Tokyo Institute of Technology
Email skondoh@bio.titech.ac.jp
Tel +81-45-924-5800
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016