Life Science and Technology News
【Labs spotlight】 Matsuda Laboratory
Green chemistry by enzymes for organic synthesis
The Department has a variety of laboratories for Life Science and Technology, in which cutting-edge innovative research is being undertaken not only in basic science and engineering but also in the areas of medicine, pharmacy, agriculture, and multidisciplinary sciences.
This "Spotlight" series features a laboratory from the Department and introduces you to the laboratory's research projects and outcomes. This time we focus on Matsuda Laboratory.

Areas of Supervision
Primary/Life Science and Technology
Associate Professor Tomoko Matsuda![]()
| Degree | 2000, Kyoto University Doctor of science |
|---|---|
| Areas of Research | Biocatalysis, Green Chemistry, Asymmetric Organic Synthesis. |
| Keywords | Enzyme, Alcohol dehydrogenase, Lipase, Carbon Dioxide, Supercritical Fluid, Green Chemistry, Chiral Synthesis. |
| Website | Matsuda Lab. |
Research interest
Our research interest is to promote green chemistry by using biocatalytic reactions; for example, asymmetric reduction of ketones by alcohol dehydrogenase, kinetic resolutions of racemic alcohol by lipase using CO2-based solvents, and carboxylation using decarboxylase using CO2 as a solvent. Because of homo-chirality in biology on the earth, for instance amino acids which are composed of only left-handed or L-isomer, the discovery and development of stereoselective organic synthetic methods for pharmaceutical and agrochemicals are very important. Chemical and biological catalysts have been developed throughout several decades. To synthesize them in environmentally friendly methods, enzymes are being used as catalysts.
1. Biocatalysis using CO2
We has utilized pressurized CO2 (liquid or supercritical CO2), which has numerous positive impacts on green chemistry, as an excellent platform for biocatalysis. It was found that pressurized CO2 is superior to conventional organic solvents for lipase-catalyzed reactions. We also achieved waste-minimization (E-factor < 0.3) in large-scale biosynthesis of chiral compounds with a continuous reactor using pressurized CO2 fluid.
Furthermore, we combined the volume-expansion capability of CO2 with a bio-based liquid intoCO2-expanded bio-based liquids as sustainable and efficient reaction media for biocatalysis (Figure 1). The lipase-catalysed transesterification in this new solvent systems resulted in increased biocatalytic activity, especially with bulky substrates such as rac-1-adamantylethanol. Currently, we are pursuing to elucidate the mechanism of the acceleration in enzymatic reactions caused by CO2. By these studies of green chemistry using enzyme and pressurized CO2, we hope to fulfill the role of scientists, to protect the environment for a sustainable future.
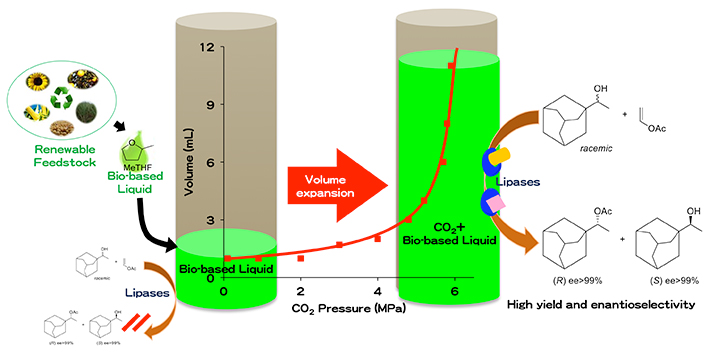
Figure 1 Expansion with CO2 tunes bio-based solvents into favorable platforms for biocatalysis
2. Biocatalytic asymmetric reduction
We have found that strains of Geotrichum candidum have many robust oxidoreductases with extremely excellent stereoselectivity. The excellent enantioselectivity can be achieved even when very challenging bulky-bulky ketones are used (Figure 2). Moreover, mutation of one key residue located within the substrate binding pocket has expanded the substrate specificity and altered the enantiopreference of this enzyme completely. Investigation of a detailed interaction between mutated residue in the binding pocket and the substrate through structural study will contribute in solving the reaction mechanism of this oxidoreductase and other oxidoreductases in general.
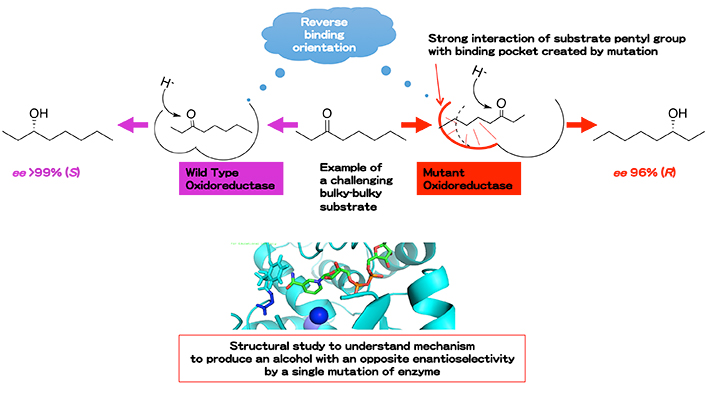
Figure 2 Biocatalytic asymmetric reduction with excellent enantioselectivity
Selected publications
1. Biocatalysis using CO2 as solvent or substrate
- [1] CO2-expanded bio-based liquids as novel solvents for enantioselective biocatalysis, H. N. Hoang, Y. Nagashima, S. Mori, H. Kagechika, T. Matsuda, Tetrahedron 2017, 73, 2984-2989.
- [2] Expanding substrate scope of lipase-catalyzed transesterification by the utilization of liquid carbon dioxide, H. N. Hoang, T. Matsuda, Tetrahedron 2016, 72, 7229-7234.
- [3] High-efficiency and minimum-waste continuous kinetic resolution of racemic alcohols by using lipase in supercritical carbon dioxide, T. Matsuda, K. Watanabe, T. Harada, K. Nakamura, Y. Arita, Y. Misumi, S. Ichikawa, T. Ikariya, Chem. Commun. 2004, 2286-2287.
- [4] Biocatalytic reduction of ketones by a semi-continuous flow process using supercritical carbon dioxide, T. Matsuda, K. Watanabe, T. Kamitanaka, T. Harada, K. Nakamura, Chem. Commun. 2003, 1198-1199.
- [5] Conversion of pyrrole to pyrrole-2-carboxylate by cells of B. megaterium in supercritical CO2, T. Matsuda, Y. Ohashi, T. Harada, R. Yanagihara, T. Nagasawa, K. Nakamura, Chem. Commun. 2001, 2194-2195.
- [6] Alcohol dehydrogenase is active in supercritical carbon dioxide, T. Matsuda, T. Harada, K. Nakamura, Chem. Commun. 2000, 1367-1368.
2. Biocatalytic asymmetric reduction
- [1] Crystallization and preliminary crystallographic analysis of acetophenone reductase from Geotrichum candidum NBRC 4597, Y. Sugiyama, M. Senda, T. Senda, T. Matsuda, Acta Crystallogr. F71, 2015, 320-323.
- [2] Two classes of enzymes of opposite stereochemistry in an organism: one for fluorinated and another for non-fluorinated substrates, T. Matsuda, T. Harada, N. Nakajima, T. Itoh, K. Nakamura, J. Org. Chem. 2000, 65, 157-163.
- [3] Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum, K. Nakamura, T. Matsuda, J. Org. Chem. 1998, 63, 8957-8964.
3. Reviews and book edition
- [1] Future directions in biocatalysis 2nd edition, T. Matsuda, Ed. Elsevier (under planning)
- [2] Future directions in biocatalysis, T. Matsuda, Ed. Elsevier, Amsterdam, 2007.
- [3] Chapter 11. Enzymatic asymmetric reduction of carbonyl compounds, T. Matsuda, R. Yamanaka, K. Nakamura, In Green Biocatalysis, Ed. R. N. Patel, Wiley & Sons, 2016, p 307-330.
- [4] Recent progress in biocatalysis for asymmetric oxidation and reduction,T. Matsuda, R. Yamanaka, K. Nakamura, Tetrahedron: Asymmetry, 2009, 20, 513-557.
- [5] Organic synthesis using enzymes in supercritical carbon dioxide,T. Matsuda, T. Harada, K. Nakamura, Green Chem. 2004, 6, 440-444.
- A message for prospective graduate students
-
Let's enjoy science!! It is most important to do research to discover something new, which may be trivial, to contribute solutions to environmental problems. We are studying enzymatic reactions to construct new organic synthesis reactions.
Be part of a delightful lab team!! Teamwork is also essential to produce unique and interesting research results. Therefore, we try to make a happy, peaceful, and cooperative group. Currently, we have 12 students with great individualities from 4 different countries. Please feel free to contact me or our members for more information.
Contact
Associate Professor Tomoko Matsuda
Room 913, 914, 915, 921, J3 building, Suzukakedai campus
Email tmatsuda@bio.titech.ac.jp
*Find more about the lab and the latest activities at the lab site![]() .
.
*May 1, 2025:Some of the content has been updated with the latest information.