Life Science and Technology News
【Labs spotlight】 Kamachi Laboratory
Elucidation of physiological role of metal ions
The Department has a variety of laboratories for Life Science and Technology, in which cutting-edge innovative research is being undertaken not only in basic science and engineering but also in the areas of medicine, pharmacy, agriculture, and multidisciplinary sciences.
This "Spotlight" series features a laboratory from the Department and introduces you to the laboratory's research projects and outcomes. This time we focus on Kamachi Laboratory.

Areas of Supervision
Primary/Life Science and Technology
Secondary/Science and Technology for health Care and Medicine, Human Centered Science and Biomedical Engineering
Professor Toshiaki Kamachi![]()
| Degree | PhD 1995, Tokyo Institute of Technology |
|---|---|
| Areas of Research | Bioinorganic Chemistry, Photochemistry, Bioengineering. |
| Keywords | Metalloprotein, Photoenergy conversion, Oxygen imaging. |
| Website | Toshiaki Kamachi |
Research interest
Life depends not only on organic compounds but also on inorganic compounds. For example, metalloproteins are important class of proteins that contain metal ions as cofactors. About half of all known proteins contain metal cofactors. These metal ions are indispensable constituents to protein function in photosynthesis, electron transport, dioxygen transport and so forth. Metal complexes also introduce to biological system for drug or diagnostic purpose. The interests of our laboratory are elucidation of the function metal ions in biological system. We are focused on the characterization and application of metalloproteins such as methane monooxygenase or hydrogenase. Methane monooxygenase contains copper or iron ions, catalyzes methane oxidation to methanol under ambient conditions. On the other hand, hydrogenase contains nickel and iron ions, catalyzes reversible oxidation of hydrogen. These enzymes can be used for energy transduction using light energy. Synthetic phosphorescent metal complexes can be used for dioxygen sensor. We applied platinum porphyrin for dioxygen measurement inside single cell under confocal microscope. Elucidation of dioxygen metabolism is the one of our interest.
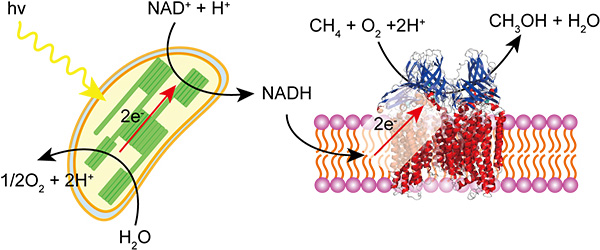
Photo-induced methanol production from methane with chloroplast and methane monooxygenase
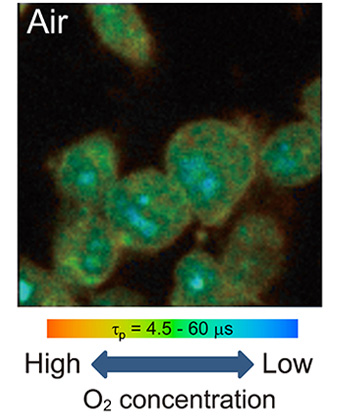
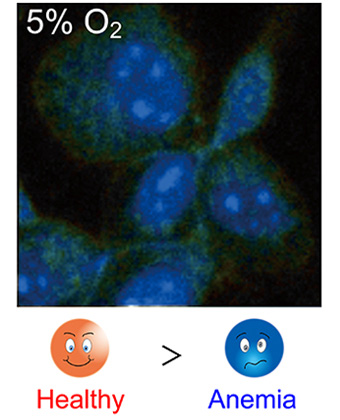
Oxygen concentration imaging inside a single cell by phosphorescence lifetime measurement
Research findings
Selected publications
- [1] M. Tsukada, T. Nakashima, T. Kamachi, Y. Niwano, Prooxidative Potential of Photo-Irradiated Aqueous Extracts of Grape Pomace, a Recyclable Resource from Winemaking Process. PLoS ONE 11, e0158197 (2016).
- [2] T. Haruyama, T. Namise, N. Shimoshimizu, S. Uemura, Y. Takatsuji, M. Hino, R. Yamasaki, T. Kamachi, M. Kohno, Non-catalyzed one-step synthesis of ammonia from atmospheric air and water. Green Chemistry 18, 4536-4541 (2016).
- [3] H. Kurokawa, H. Ito, M. Inoue, K. Tabata, Y. Sato, K. Yamagata, S. Kizaka-Kondoh, T. Kadonosono, S. Yano, M. Inoue, T. Kamachi, High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime. Sci. Rep. 5, 10657, (2015).
- [4] T. Kobayashi, T. Yoshida, T. Fujisawa, Y. Matsumura, T. Ozawa, H. Yanai, A. Iwasawa, T. Kamachi, K. Fujiwara, M. Kohno, N. Tanaka, A metabolomics-based approach for predicting stages of chronic kidney disease. Biochem. Biophys. Res. Commun. 445, 412-416 (2014).
- [5] Kudo, F.; Hoshi, S.; Kawashima, T.; Kamachi, T.; Eguchi, T., Characterization of a Radical S-Adenosyl-l-methionine Epimerase, NeoN, in the Last Step of Neomycin B Biosynthesis. J. Am. Chem. Soc. 2014, 136 (39), 13909-13915.
- [6] Kobayashi, T.; Matsumura, Y.; Ozawa, T.; Yanai, H.; Iwasawa, A.; Kamachi, T.; Fujiwara, K.; Tanaka, N.; Kohno, M., Exploration of novel predictive markers in rat plasma of the early stages of chronic renal failure. Anal. Bioanal. Chem. 2014, 406 (5), 1365-1376.
- [7] Ito, H.; Mori, F.; Tabata, K.; Okura, I.; Kamachi, T., Methane hydroxylation using light energy by the combination of thylakoid and methane monooxygenase. RSC Advances 2014, 4 (17), 8645-8648.
- [8] Tabata, K.; Hida, F.; Kiriyama, T.; Ishizaki, N.; Kamachi, T.; Okura, I., Measurement of soil bacterial colony temperatures and isolation of a high heat-producing bacterium. BMC Microbiol. 2013, 13 (1), 56-62.
- [9] Ito, H.; Kamachi, T.; Yashima, E., Specific surface modification of the acetylene-linked glycolipid vesicle by click chemistry. Chem. Commun. 2012, 48 (45), 5650-5652.
- [10] Yamamura, T.; Suzuki, S.; Taguchi, T.; Onoda, A.; Kamachi, T.; Okura, I., Porphyrin arrays responsive to additives. Fluorescence tuning. J. Am. Chem. Soc. 2009, 131 (33), 11719-11726.
- [11] Saito, T.; Asakura, N.; Kamachi, T.; Okura, I., Oxygen concentration imaging in a single living cell using phosphorescence lifetime of Pt-porphyrin. J. Porphyr. Phthalocya. 2007, 11 (3-4), 160-164.
- [12] Iida, S.; Asakura, N.; Tabata, K.; Okura, I.; Kamachi, T., Incorporation of unnatural amino acids into cytochrome c3 and specific viologen binding to the unnatural amino acid. ChemBioChem 2006, 7 (12), 1853-1855.
- [13] Ihara, M.; Nishihara, H.; Yoon, K. S.; Lenz, O.; Friedrich, B.; Nakamoto, H.; Kojima, K.; Honma, D.; Kamachi, T.; Okura, I., Light-driven hydrogen production by a hybrid complex of a [NiFe]-hydrogenase and the cyanobacterial photosystem I. Photochem. Photobiol. 2006, 82 (3), 676-682.
- [14] Ihara, M.; Nakamoto, H.; Kamachi, T.; Okura, I.; Maeda, M., Photoinduced hydrogen production by direct electron transfer from photosystem I cross-linked with cytochrome c3 to [NiFe]-hydrogenase. Photochem. Photobiol. 2006, 82 (6), 1677-1685.
- [15] Asakura, N.; Kamachi, T.; Okura, I., Direct monitoring of the electron pool effect of cytochrome c3 by highly sensitive EQCM measurements. J. Biol. Inorg. Chem. 2004, 9 (8), 1007-1016.
Contact
Professor Toshiaki Kamachi
Room 301A, M6 building, Ookayama campus
E-mail : tkamachi@bio.titech.ac.jp
*Find more about the lab and the latest activities at the lab site![]() (Japanese).
(Japanese).
*May 1, 2025:Some of the content has been updated with the latest information.