Life Science and Technology News
A Winding Road: Mapping How Singlet Oxygen Molecules Travel Along DNA Strands
A recent study has unveiled with unprecedented detail how singlet oxygen molecules diffuse along double strand DNAs, paving the way to more effective nucleic acid-targeting photodynamic therapy (PDT). A research team at Tokyo Tech used a novel photosensitizer and custom-made DNA sequences to shed light on the optimal position to anchor the photosensitizer to achieve the best oxidizing effect. This could help make this type of PDT more lethal to cancer cells.
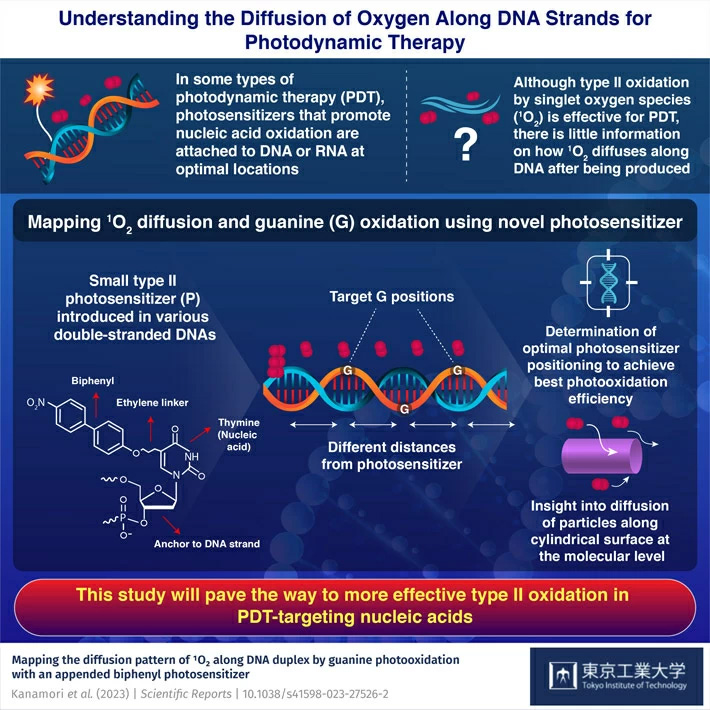
Nucleic acid-targeting photodynamic therapy (PDT) is a promising type of targeted therapy that is being actively researched. This treatment relies on special photosensitizers, a type of drug that binds at specific locations in a cell's DNA. Once bound, the cells are irradiated at a precise frequency, which in turn causes the photosensitizer to produce reactive oxygen species (ROS) or singlet oxygen (1O2) molecules. These molecules tend to oxidize nearby nucleic acids, damaging the genetic material and ultimately killing the irradiated cell.
Although the overall process may sound straightforward, there are still many hurdles to overcome before this type of PDT is good enough for clinical practice. One of them is that even though type II oxidation (the one caused by 1O2) has certain advantages over type I oxidation (the one caused by ROS), there is very little information on how far 1O2 molecules can reach once generated. Because of this knowledge gap, it is difficult to decide which location in the DNA should be targeted to achieve the best effect.
Fortunately, in a recent study, a research team from Tokyo Institute of Technology, Japan, sought to address this issue. As described in their paper published in Scientific Reports![]() , the team, led by Professor Hideya Yuasa, employed an innovative approach to study how 1O2 propagates along double strand DNA and how well it can oxidize nearby guanine (G) sites depending on the distance to the photosensitizer.
, the team, led by Professor Hideya Yuasa, employed an innovative approach to study how 1O2 propagates along double strand DNA and how well it can oxidize nearby guanine (G) sites depending on the distance to the photosensitizer.
The researchers prepared a series of double strand DNA molecules with multiple G sites at different locations relative to the place where the photosensitizer anchored itself. Then, after irradiating the DNA, they analyzed which G sites were more consistently oxidated. Worth noting, the photosensitizer they used was designed based on previous studies also led by Prof. Yuasa. In this case, the photosensitizer consisted of a biphenyl group 'hanging' from a short, freely rotatable linker bound to thymine, one of the building blocks of DNA. What made this photosensitizer particularly useful for this study were its small size—which ensured that 1O2 diffusion was not significantly disturbed—and its remarkably high tendency to produce 1O2 exclusively upon irradiation compared to other photosensitizers.
After several experiments followed by theoretical analysis, the team determined the optimal distances to the photosensitizer to achieve the highest oxidation of G. Moreover, they shed light on certain electronic mechanisms that quench the oxidation of G at positions closer to the photosensitizer. "Our study provides information about how 1O2 travels along DNA duplexes in more detail than ever, thereby offering clues on how to overcome the low reactivity of type II photooxidation in nucleic acid targeting PDT," remarks Professor Yuasa.
Overall, the findings of this work put us one step closer to next-generation PDT, which could become a great tool to fight cancer. "Our mapping of the diffusion of 1O2 along DNA duplexes will be important to develop efficient and selective photosensitizer agents for PDT," concludes Professor Yuasa, "It also serves as an experimental demonstration of the diffusion of particles along a cylindrical surface at the molecular level."
Keep an eye out for further advances in this technology, as it may soon start saving lives!
- Reference
| Authors : | Takashi Kanamori*, Shota Kaneko, Koji Hamamoto, and Hideya Yuasa* |
|---|---|
| Title : | Mapping the diffusion pattern of 1O2 along DNA duplex by guanine photooxidation with an appended biphenyl photosensitizer |
| Journal : | Scientific Reports |
| DOI : | 10.1038/s41598-023-27526-2 |
| Affiliations : | School of Life Science and Technology, Tokyo Institute of Technology |
| * Corresponding authors' emails: | kanamori.t.ac@m.titech.ac.jp; hyuasa@bio.titech.ac.jp |
- 【Labs spotlight】 Yuasa Laboratory | Life Science and Technology News
- Yuasa Laboratory (Japanese)
- Takashi Kanamori | Researcher Finder - Tokyo Tech STAR Search
- Hideya Yuasa | Researcher Finder - Tokyo Tech STAR Search
- Department of Life Science and Technology, School of Life Science and Technology
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Further Information
Professor Hideya Yuasa
School of Life Science and Technology, Tokyo Institute of Technology
E-mail : hyuasa@bio.titech.ac.jp