Life Science and Technology News
Biofuel produced by microalgae
Biofuel produced by microalgae: The perimeter of lipid droplet in their cells is an important place for oil synthesis
Scientists at Tokyo Institute of Technology have identified unique lysophosphatidic acid acyltransferases as being the central enzymes for triacylglycerol synthesis by oleaginous alga Nannochloropsis, thus uncovering the mechanisms of biofuel production in microalgae.
In the modern society, energy generation heavily relies on fossil fuels, which, however, lead to environmental pollution and depletion of non-renewable resources. Photosynthetic organisms such as plants and green algae can transform atmospheric carbon dioxide into carbon storage molecules, especially oils such as triacylglycerols (TAGs), which can be used as biofuels. In this context, microalgae provide advantages of high oil content and growth in extreme environments, including high salinity, temperature, or pH.
Nannochloropsis (Figure 1) is a genus of microalgae which can accumulate TAGs up to 50% of dry weight; however, the mechanisms underlying their oleaginous trait are largely unknown.
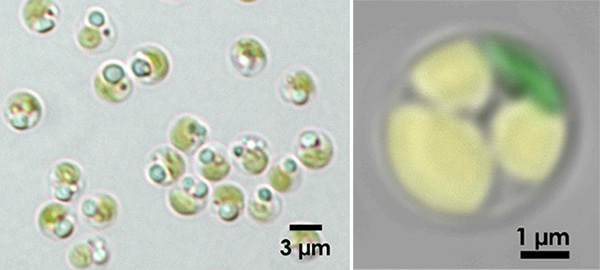
Figure 1. Oleaginous microalga Nannochloropsis
Left panel: Light microscopic image of Nannochloropsis. Lipid droplets look slightly bluish by light refraction.
Right panel: Fluorescence micrograph of that accumulated a large amount of oils. With color-coding, green indicates chloroplasts, and yellow lipid droplets.
Scientists from Tokyo Institute of Technology lead by Professor Hiroyuki Ohta, who teaches Life Science and Technology, have addressed this problem by investigating lipid metabolism in Nannochloropsis oceanica. TAGs are synthesized in the extraplastidic Kennedy pathway through sequential addition of three fatty acyl moieties to the glycerol backbone. Among the participating enzymes, the scientists focused on four lysophosphatidic acid acyltransferases (LPATs 1-4) responsible for the addition of fatty acids at position 2.
They found that phylogenetically, LPAT1 and LPAT2 belong to different subfamilies, while LPAT3 and LPAT4 have a close evolutionary relationship. Accordingly, these enzymes appeared to have distinct functional activities as revealed by using mutant strains of N. oceanica lacking either one or two of the four LPATs. Thus, LPAT1 was found to mainly participate in the synthesis of membrane lipids, while LPAT4 was responsible for TAG biosynthesis, and LPAT2 and LPAT3 contributed to both processes (Figure 2).
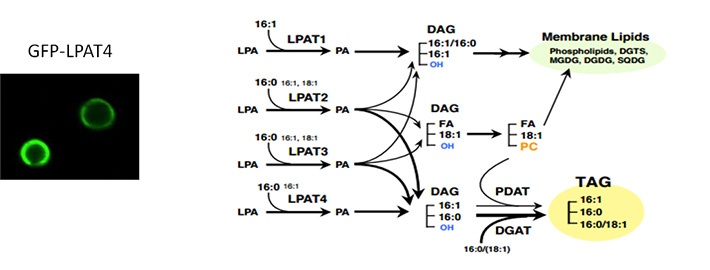
Figure 2. Model of de novo lipid biosynthetic pathway mediated by the four LPATs in Nannochloropsis
Left panel: Localization of LPAT4 labeled with green fluorescent protein (GFP) to the perimeter of lipid drops. Right panel: A model of LPAT activity in Nannochloropsis. LPAT1 participates in the biosynthesis of membrane lipids, while LPAT2 contribute to TAG biosynthesis in the ER, and LPAT3 and 4 in the perimeter of lipid droplets.
LPATs were labeled with fluorescent tags and their intracellular location was examined using confocal microscopy. While LPAT1 and LPAT2 showed a typical ER localization pattern, LPAT3 and LPAT4 were observed specifically at the perimeter of lipid droplets (LDs) (Figure 2), which was likely due to the presence of long (30-40 residues) hydrophobic domains in their structures that enable anchoring to the LD surface.
Based on their results, the scientists suggest that for LD formation, LPAT2 is mainly involved in the initial TAG synthesis in the ER, and LPAT3 and LPAT4 localize on LD surface in the periphery and contribute to further growth of LDs.
Thus, the study of Professor Ohta and his colleagues provide direct evidence that the oleaginous trait of Nannochloropsis is supported by LPATs in the perimeter of LDs.
Reference
| Authors: | Takashi Nobusawaa, d, Koichi Horia, d, Hiroshi Moria, d, Ken Kurokawab, c, d, Hiroyuki Ohtaa,b,d |
|---|---|
| Title of original paper: | Differently Localized Lysophosphatidic Acid Acyltransferases Crucial for Triacylglycerol Biosynthesis in the Oleaginous Alga Nannochloropsispaper |
| Journal: | The Plant Journal |
| DOI : | 10.1111/tpj.13512 |
| Affiliations : |
a School of Life Science and Technology, Tokyo Institute of Technology b Earth-Life Science Institute, Tokyo Institute of Technology c Center for Information Biology, National Institute of Genetics d CREST, JST |
- Genetic manipulation for algal biofuel production | Tokyo Tech News
- A new transgenic plant to produce biofuel | Tokyo Tech News
- Plant fertility - how hormones get around | Tokyo Tech News
- Lipid accumulation enhancement achieved using nutrition starvation-inducible promoter in algae | Tokyo Tech News
- Genome analysis reveals how algae evolved into land plants | Tokyo Tech News
- Labs spotlight #12 - Ohta and Shimojima Laboratory -
- Researcher Profile | Tokyo Tech STAR Search - Hiroyuki Ohta
- Researcher Profile | Tokyo Tech STAR Search - Takashi Nobusawa
- National Institute of Genetics
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Further information
Professor Hiroyuki Ohta
School of Life Science and Technology
Email hohta@bio.titech.ac.jp
Tel +81-45-924-5736