Life Science and Technology News
【Labs spotlight】 Mihara and Tsutsumi Laboratory
Peptide Engineering & Chemical Biology
The Department has a variety of laboratories for Life Science and Technology, in which cutting-edge innovative research is being undertaken not only in basic science and engineering but also in the areas of medicine, pharmacy, agriculture, and multidisciplinary sciences.
This "Spotlight" series features a laboratory from the Department and introduces you to the laboratory's research projects and outcomes. This time we focus on Mihara and Tsutsumi Laboratory.
*March 31, 2024:Professor Mihara retired from Tokyo Tech.

Areas of Supervision
Primary/Life Science and Technology
Professor Hisakazu Mihara![]() and Associate Professor Hiroshi Tsutsumi
and Associate Professor Hiroshi Tsutsumi![]()
| Degree | Professor Hisakazu Mihara : Dr.Sc. 1986, Kyushu University Associate Professor Hiroshi Tsutsumi : PhD 2004, Tokyo Institute of Technology |
|---|---|
| Areas of Research | Professor Hisakazu Mihara : Bioorganic Chemistry, Chemical Biology, Peptide Engineering Associate Professor Hiroshi Tsutsumi : Chemical Biology, Supramolecular Chemistry, Peptide Chemistry |
| Keywords | Professor Hisakazu Mihara : Designed Peptide, Peptide Biochip, Self-Assembling Peptide, Biomaterial for Cell, Peptide Phage Display Associate Professor Hiroshi Tsutsumi : Peptide, Hydrogel, Phage Display, Bioimaging |
| Website | Mihara Laboratory |
Research interest
Development of functional peptides based on peptide engineering
We design various functional peptides with characteristic structures such as α-helix and β-sheet. Main three projects are "Hydrogel materials as a scaffold for cell proliferation and differentiation", "Cell penetrating peptides (CPP) for selective drug delivery" and "Peptide ligands for detection of lectin proteins associated with virus/bacteria infection to cells".
1. Development of self-assembling peptides and application to nanomaterials and biomaterials
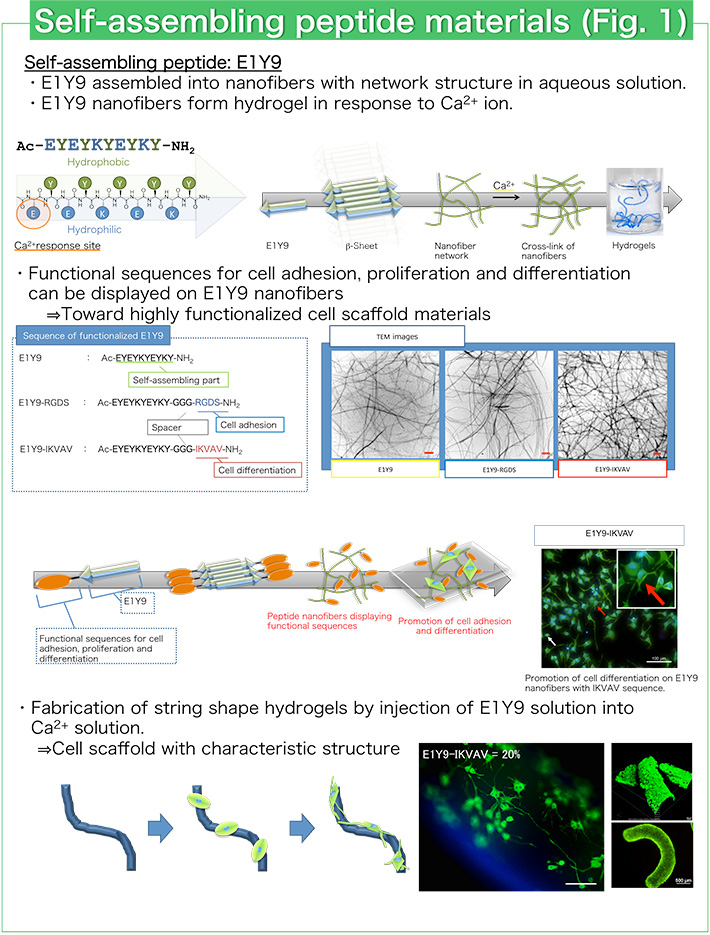
Peptides that spontaneously assemble into various and precise structures have been designed using electrostatic interaction, hydrophobic interaction and hydrogen bond formation. We have designed a self-assembling peptide E1Y9 that can assemble into nanofibers with a β-sheet structure in aqueous solution (Fig. 1). E1Y9 can form hydrogels in response to calcium ion (Ca2+). E1Y9 hydrogels have similar properties to natural hydrogels like collagen and give suitable environments for cell proliferation. Therefore, E1Y9 hydrogels are expected to be good biomaterials for cell culture and regenerative medicine. For example, E1Y9 hydrogel with string shape is useful for the culture and differentiation control of neuronal cells (Fig. 1). Culture and differentiation control of various cells such as osteoblast cells and stem cells have been performed using E1Y9 hydrogels.
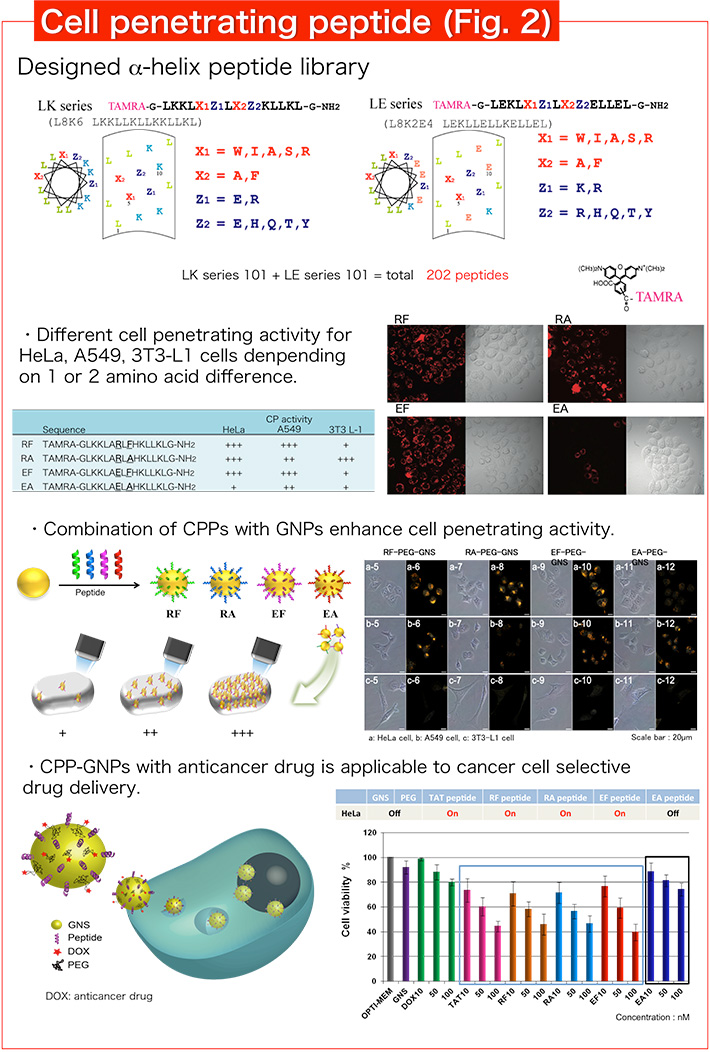
2. Development of α-helix peptides with cell penetrating activity and application to drug delivery
Recently, various peptides with cell penetrating activity have been designed. We designed an amphiphilic α-helix peptide library by systematic replacement of amino acids, and found various cell penetrating peptides (CPPs) from our library (Fig. 2). In particular, selective CPPs to cancer cells are found and expected to be a useful drug delivery tool. We combined cancer cell selective CPP and gold nanoparticles (GNPs) to produce hybrid materials for drug delivery. CPP-GNPs with anticancer drug showed selective anticancer activity corresponding to cell penetrating activity of α-helix peptides. In addition, we are screening CPPs with further cancer cell penetrating activity and selectivity by the detail analysis of cell penetrating activity.
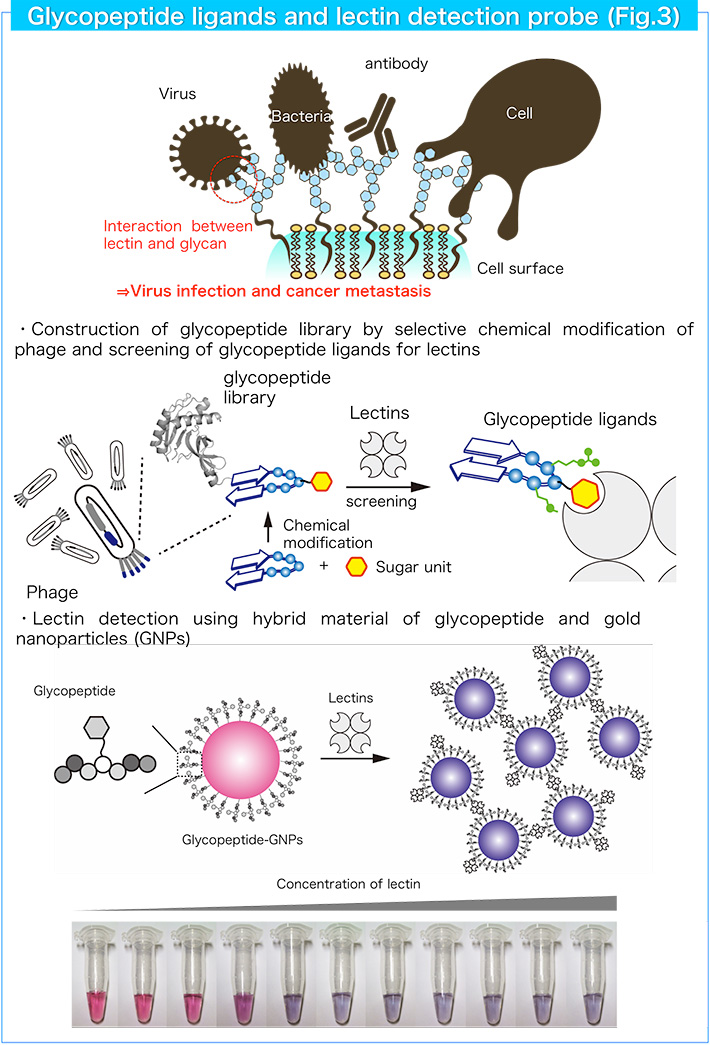
3. Development of glycopeptide ligands for lectins and lectin detection probes
Lectins are carbohydrate-binding proteins and recognize mono/oligosaccharides with high selectivity. As various lectins and glycoconjugates such as glycoproteins and glycolipids are found on the cell surface, the lectin-glycoconjugate interaction is associated with various life events including cell-cell interactions, pathogen (virus) infection and tumor metastasis. Therefore, the selective detection of lectins and glycoconjugates is important to disclose the complex biological processes mediated by lectins. We designed a glycopeptide platform as a new candidate to produce various lectin ligands. Combination of a sugar unit with various peptide sequences will give useful ligands comparable to native glycans. We designed and constructed glycopeptide libraries using a genetic engineering method and a chemical modification method, and found useful glycopeptide ligands with high binding affinity and selectivity (Fig. 3). In addition, we developed optical lectin detection probes by combining glycopeptide ligands with gold nanoparticles (Fig. 3).
Research result
Selected publications
- [1] K. Fukunaga, H. Tsutsumi, H. Mihara. Cell Differentiation on Disk- and String-Shaped Hydrogels Fabricated from Ca2+-responsive Self-Assembling Peptides. Biopolymers (Pept. Sci), 2016, 106, 476-483.
- [2] H. Tsutsumi, K. Nakano, H. Mihara. Dihydrofolate Reductase Inhibitory Peptides Screened from a Structured Design β-Loop Peptide Library Displayed on Phage. Mol. BioSyst., 2015, 11, 2713-2716.
- [3] J. Yang, T. Furuta, M. Sakurai, H. Tsutsumi, H. Mihara. A Computational Study of the Interaction of Amphiphilic α-Helical Cell-Penetrating Peptides with Heparan Sulfate. Bull. Chem. Soc. Jpn., 2014, 87, 1074-1082.
- [4] K. Usui, T. Kikuchi, K. Kikuchi, M. Mie, E. Kobatake, H. Mihara. Cellular Differentiation Assessments by Measuring the Degree of Cellular Internalization and Membrane Adsorption Using Designed Peptides. Bioorg Med Chem Lett, 2014, 24, 4129-4131.
- [5] J. Yang, H. Tsutsumi, T. Furuta, M. Sakurai, H. Mihara. Interaction of Amphiphilic α-Helical Cell-Penetrating Peptides with Heparan Sulfate. Org. Biomol. Chem., 2014, 12, 4673-4681.
- [6] H. Park, H. Tsutsumi, H. Mihara. Cell-Selective Intracellular Drug Delivery Using Doxorubicin and α-Helical Peptides Conjugated to Gold Nanoparticles. Biomaterials, 2014, 35, 3480-3487.
- [7] K. Usui, T. Kikuchi, KY. Tomizaki, T. Kakiyama, H. Mihara. A Novel Array Format for Monitoring Cellular Uptake Using a Photo-Cleavable Linker for Peptide Release. Chem. Commun., 2013, 49, 6394-6396.
- [8] H. Tsutsumi, H. Mihara. Self-Assembly of Designed Peptides and Their Nanomaterials Applications. Amino Acids, Peptides and Proteins, ed. E. Farkas and M. Ryadnov, Royal Society of Chemistry Publishing, Cambridge, UK, 2013, vol. 38, pp. 122-150.
- [9] H. Tsutsumi, H. Mihara. Soft Materials Based on Designed Self-Assembling Peptides: from Design to Application. Mol. BioSyst., 2013, 9, 609-617.
- [10] K. Fukunaga, H. Tsutsumi, H. Mihara. Self-Assembling Peptide Nanofibers Promoting Cell Adhesion and Differentiation. Biopolymers (Pept. Sci), 2013, 100, 731-737.
- [11] K. Arai, H. Tsutsumi, H. Mihara. A Monosaccharide-Modified Peptide Phage Library for Screening of Ligands to Carbohydrate-Binding Proteins. Bioorg. Med. Chem. Lett., 2013, 23, 4940-4943.
- [12] H. Park, H. Tsutsumi, H. Mihara. Cell Penetration and Cell-Selective Drug Delivery Using α-Helix Peptides Conjugated with Gold Nanoparticles. Biomaterials, 2013, 34, 4872-4879.
- [13] K. Usui, T. Kikuchi, M. Mie, E. Kobatake, H. Mihara. Systematic Screening of the Cellular Uptake of Designed Alpha-Helix Peptides. Bioorg. Med. Chem., 2013, 21, 2560-2567.
- [14] H. Tsutsumi, H. Ohkusa, H. Park, T. Takahashi, H. Yuasa, H. Mihara. Gold Nanoparticles Conjugated with Monosaccharide-modified Peptide for Lectin Detection. Bioorg. Med. Chem. Lett., 2012, 22, 6825-6827.
- [15] T. Sawada, M. Tsuchiya, T. Takahashi, H. Tsutsumi, H. Mihara. Cell-Adhesive Hydrogels Composed of Peptide Nanofibers Responsive to Biological Ions. Polymer J., 2012, 44, 651-657.
- [16] T. Sawada, H. Mihara. Dense Surface Functionalization Using Peptides that Recognize Differences in Organized Structures of Self-assembling Nanomaterials. Mol. BioSyst., 2012, 8, 1264-1274.
- [17] A. Syahir, K. Kajikawa, H. Mihara. Sensitive Detection of Small Molecule-Protein Interactions on a Metal-Insulator-Metal Label-Free Biosensing Platform. Chem. Asia. J., 2012, 7, 1867-1874.
- [18] K. Usui, T. Kakiyama, K. Tomizaki, M. Mie, E. Kobatake, H. Mihara. Cell Fingerprint Patterns Using Designed α-Helical Peptides to Screen for Cell-Specific Toxicity. Bioorg. Med. Chem. Lett., 2011, 21, 6281-6284.
- [19] T. Sawada, K. Ishiguro, T. Takahashi, H. Mihara. A Novel β-loop Scaffold of Phage-Displayed Peptides for Highly Specific Affinities. Mol. BioSyst., 2011, 7, 2558-2562.
- [20] A. Miyachi, T. Takahashi, S. Matsumura, H. Mihara. Peptide Nanofibers Modified with a Protein by Using Designed Anchor Molecules Bearing Hydrophobic and Functional Moieties. Chem. Eur. J., 2010, 16, 6644-6650.
- [21] T. Sawada, T. Takahashi, H. Mihara. Affinity-Based Screening of Peptides Recognizing Assembly States of Self-Assembling Peptide Nanomaterials. J. Am. Chem. Soc., 2009, 131, 14434-14441.
- [22] K. Usui, K.-Y. Tomizaki, H. Mihara. Screening of α-Helical Peptide Ligands Controlling a Calcineurin-phosphatase Activity. Bioorg. Med. Chem. Lett., 2007, 17, 167-17
- [23] S. Matsumura, S. Uemura, H. Mihara. Metal-triggered Nanofiber Formation of His-containing β-sheet Peptide. Suplamolc. Chem., 2006, 18, 397-403.
- [24] K. Usui, T. Ojima, K.-Y. Tomizaki,, H. Mihara. A Designed Glycopeptide Array for Characterization of Sugar-Binding Proteins Toward a Glycopeptide Chip Technology. NanoBiotechnology, 2005, 1, 191-200.
- [25] S. Matsumura, S. Uemura, H. Mihara. Construction of biotinylated peptide nanotubes for arranging proteins. Mol. BioSyst., 2005, 1, 146-148.
- Research Laboratories and Subjects
- Messages from heads of Schools, ILA, and IIR established in April 2016 | Tokyo Tech News
Contact
Professor Hisakazu Mihara
Room 801, B1 building, Suzukakedai campus
Email hmihara@bio.titech.ac.jp
Associate Professor Hiroshi Tsutsumi
Room 802, B1 building, Suzukakedai campus
Email htsutsum@bio.titech.ac.jp
*Find more about the lab and the latest activities at the lab site![]() (Japanese).
(Japanese).
*May 1, 2025:Some of the content has been updated with the latest information.