Life Science and Technology News
A new tumor suppressor gene for breast cancer in mice
Researchers at Tokyo Tech revealed a role for an X-linked protein kinase Nrk in terminating the proliferation of mammary epithelial cells during pregnancy and thereby preventing breast tumorigenesis in mice.
In recent years, the incidence of breast cancer has been increasing worldwide, and breast cancer is becoming a serious object of public concern. The onset of breast cancer is closely related to the sex hormone estrogen, and estrogen antagonists such as tamoxifen have been used as anti-breast cancer drugs. During pregnancy, the elevated blood estrogen level induces the proliferation of mammary epithelial cells, leading to the development of the mammary gland in preparation for lactation. The mammary epithelial cells eventually stop proliferation at late stages of pregnancy, impairment of which potentially results in breast tumorigenesis (Fig. 1). However, the regulatory mechanisms of mammary epithelial cell proliferation during pregnancy have been unclear.
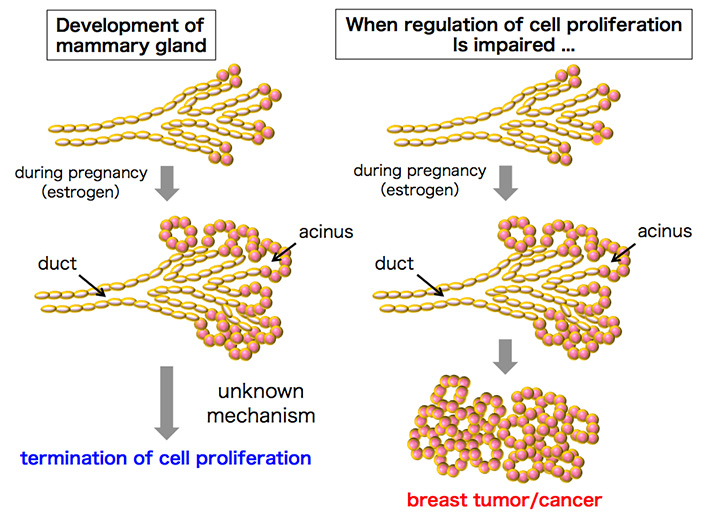
Figure 1. Proliferation of mammary epithelial cells and breast cancer
Nrk is a protein kinase encoded in the X chromosome. To study the Nrk function in vivo, Professor Masayuki Komada, who teaches Life Science and Technology, and colleagues at Tokyo Institute of Technology and Tokai University, generated mutant mice with disrupted Nrk gene, making them unable to produce the Nrk protein. During maintenance of the mutant mouse colony, the researchers noticed that the Nrk mutant mice frequently exhibited breast tumors after repetitive pregnancy/parturition. The absence of tumors in virgin Nrk mutant mice strongly suggests that the tumorigenesis is closely related to the proliferation of mammary epithelial cells during pregnancy. Histopathological examinations showed that this breast tumor is a non-invasive, relatively benign cancer (Fig. 2). In addition, the tumor was positive for estrogen receptor α (ERα) and cell proliferation marker Ki67, and negative for receptor tyrosine kinase HER2/ErbB2, suggesting that it has features like luminal B type breast cancer in humans (Fig. 3).
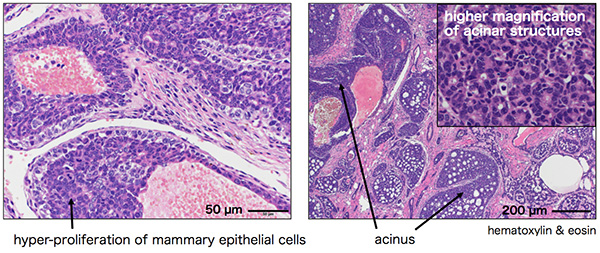
Figure 2. Histological analysis of Nrk mutant breast tumor
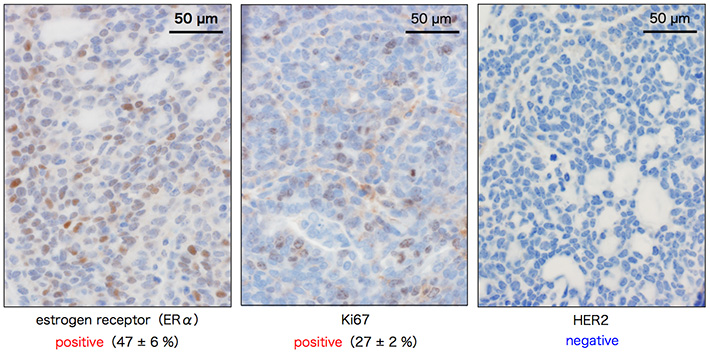
Figure 3. Subtype classification of Nrk mutant breast tumor
In Nrk mutant mammary glands, dense clusters of ERα-positive mammary epithelial cells were sometimes observed at late stages of pregnancy, which may represent the "bud" of the breast tumor (Fig. 4). While Nrk expression was undetectable in the mammary glands of wild-type mice in non-pregnant conditions, it was detected at late stages of pregnancy. This suggests that Nrk is expressed in mammary epithelial cells late in pregnancy in order to terminate their proliferation. In addition, blood estrogen levels at late stages of pregnancy were found to be about two times higher in Nrk mutant mice than in wild-type mice, suggesting that Nrk is also involved in the regulation of synthesis or secretion of estrogen. In Nrk mutant mice, therefore, mammary tumorigenesis was proposed to be triggered by a combination of dysregulation of proliferation in mammary epithelial cells and excessive blood estrogen.
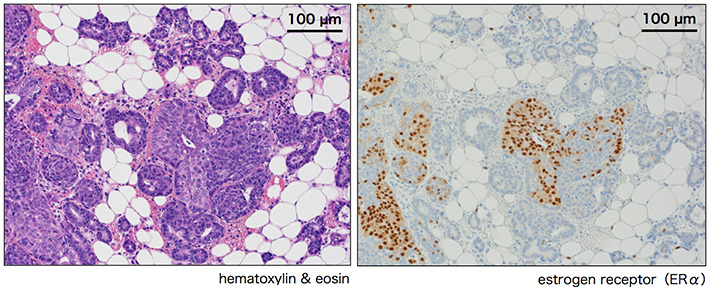
Figure 4. ERα expression of in Nrk mutant mammary epithelial cells
Collectively, these results suggest that Nrk negatively regulates the estrogen-dependent proliferation of mammary epithelial cells, the disruption of which leads to breast tumors (Fig. 5). Humans have an ortholog of the murine Nrk gene, and considering that the gene expression pattern in breast tumor in Nrk mutant mice was similar to that in human luminal B breast cancer, the findings of this study may lead to further understanding of the mechanisms of human breast cancer suppression and to advances in its diagnosis and therapy.
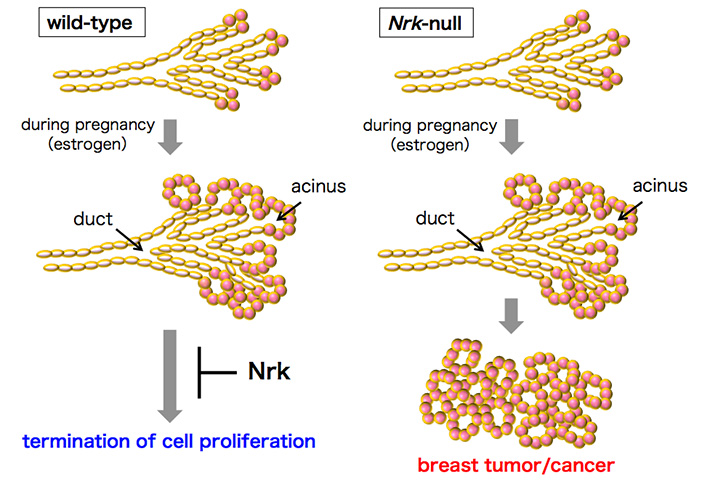
Figure 5. Impaired regulation of mammary epithelial cell proliferation in Nrk mutant mice
Reference
| Authors: | Yanagawa T1, 2, 3, Denda K1, Inatani T1, Fukushima T1, 4, Tanaka T1, Kumaki N5, Inagaki Y2, 3, and Komada M1, 4 |
|---|---|
| Title of original paper: | Deficiency of X-linked protein kinase Nrk during pregnancy triggers breast tumor in mice |
| Journal: | The American Journal of Pathology |
| DOI : | 10.1016/j.ajpath.2016.06.005 |
| Affiliations: | 1 Department of Biological Sciences, Tokyo Institute of Technology 2 Center for Matrix Biology and Medicine, Tokai University 3 Department of Regenerative Medicine, Tokai University 4 Cell Biology Unit, Institute of Innovative Research, Tokyo Institute of Technology 5 Department of Pathology, Tokai University |
- Komada Laboratory
- Researcher Profile | Tokyo Tech STAR Search - Masayuki Komada
- Genetics research demystifies fatal glandular disease | Tokyo Tech News
- Cell Biology Unit | Research Units | Institute of Innovative Research
- Department of Life Science and Technology, School of Life Science and Technology
- Institute of Innovative Research (IIR)
- Latest Research News
Further information
Professor Masayuki Komada
Cell Biology Unit, Institute of Innovative Research
Email makomada@bio.titech.ac.jp
Tel +81-45-924-5703