Materials Science and Engineering News
Frequency modulated AFM sheds light on how dipeptides help organize, immobilize and catalyze
Researchers at Science Tokyo and Nano Life Science Institute (WPI-NanoLSI), Kanazawa University observe the configuration of different dipeptides on graphite electrodes and the subsequent arrangement of catalytic hemin on them to get an idea of the factors affecting its catalytic activity.
Self-assembled peptides have shown great promise for immobilizing and exploiting enzymes in catalytic applications. However, so far little has been known as to the structures of these self-assembled peptides and how this might affect the function of the enzyme immobilized. Now researchers led by Marie Sugiyama and Yuhei Hayamizu at Institute of Science Tokyo (Science Tokyo), and Ayhan Yurtsever and Takeshi Fukuma at Kanazawa University, WPI-NanoLSI have compared the morphology and activity of hemin adsorbed on different dipeptide nanostructures using atomic force microscopy (AFM), cyclic voltammetry and H2O2 reduction reactions to see which offers the best performance and why (Figure 1).
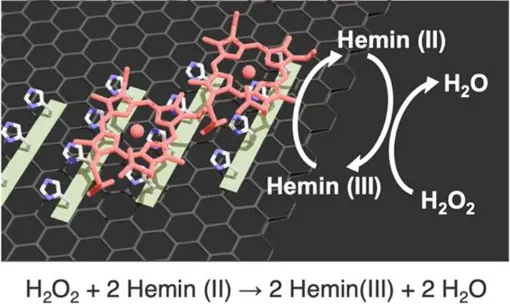
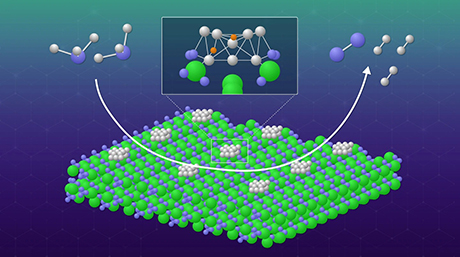
Figure 1. A schematic illustration depicting the catalytic reaction mechanism of the system. ©2025 American Chemical Society
The researchers used frequency modulated atomic force microscopy to study the structures that self-assembled from droplets of (XH)4 peptide solution on a graphite electrode, where H is histidine and X is an amino acid – either Y, L or V. Their observations indicated that dipeptides self-assemble into repeating nanostructures resembling 2D crystals, with (YH)4 exhibiting the most ordered and stable configuration (Figure 2)
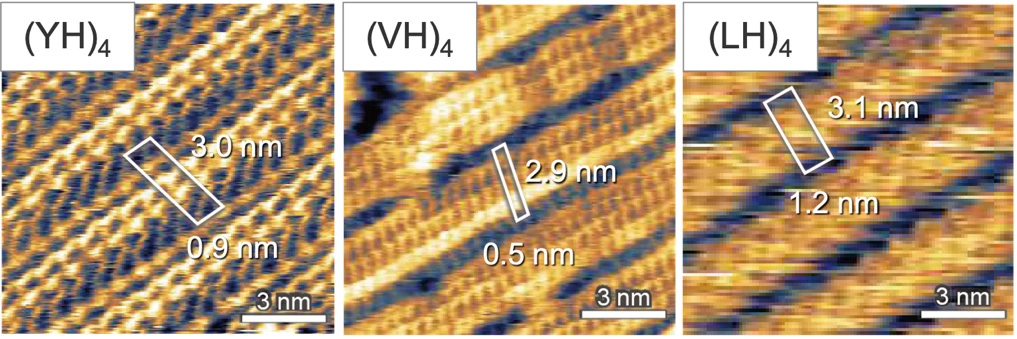
Figure 2.In situ FM-AFM images showing the unit cells of individual peptide assemblies on graphite. ©2025 American Chemical Society
They then replaced the droplet of peptide solution with a droplet of hemin solution and used AFM to observe the configuration of the hemin as it bound to the peptide structure. They found the hemin aggregated on the dipeptide structures, and further observations with high-speed AFM revealed that the hemin formed wires as well as aggregates, and that while the wires were stationary the aggregates seemed to hop along and between rows of the dipeptide (Figure 3).
The researchers used cyclic voltammetry to measure how densely hemin bound to the dipeptide structures and found that it bound most densely to (YH)4. They attribute this to the tyrosine in (YH)4, which interacts with porphyrin through π-π interactions. However, adding just porphyrin to the dipeptide bound hemin structures had little effect, from which they deduced that “the Fe atom in hemin is critical for its interaction with peptides, and that the binding is not solely driven by π−π stacking interactions,” as they report in ACS Nano. While the density of hemin binding to (LH)4 was close to that for (VH)4, they found it bound slightly more densely to (VH)4, which they attribute to the greater hydrophobicity.
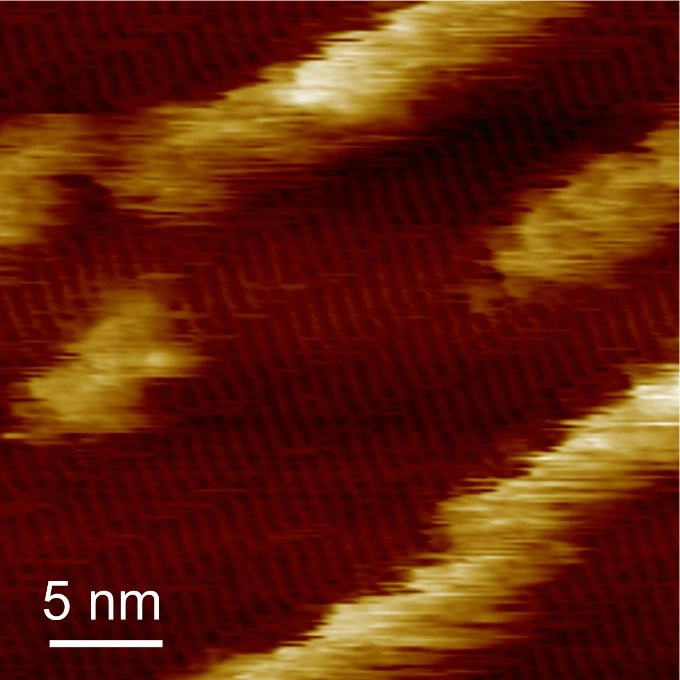
Figure 3.In situ AFM image showing the immobilization of hemin on self-assembled (YH)4 peptides, revealing the formation of hemin molecular rows along peptide lattices. ©2025 American Chemical Society
On applying a reduction current to the electrodes, the iron in hemin is reduced to the ferrous (+2) oxidation state. It can then reduce H2O2, thereby recovering its ferric oxidation state. Comparisons of how quickly the hemin bound structures reduce H2O2 revealed that hemin bound to (YH)4 had the highest catalytic activity, although this is unlikely due to the greater density of hemin at this surface since the densities for all three dipeptides were all within the same order of magnitude. Instead, the researchers suggest the greater reducing power of hemin bound to (YH)4 is on account of the more stable scaffold offered by that dipeptide (Figure 4).
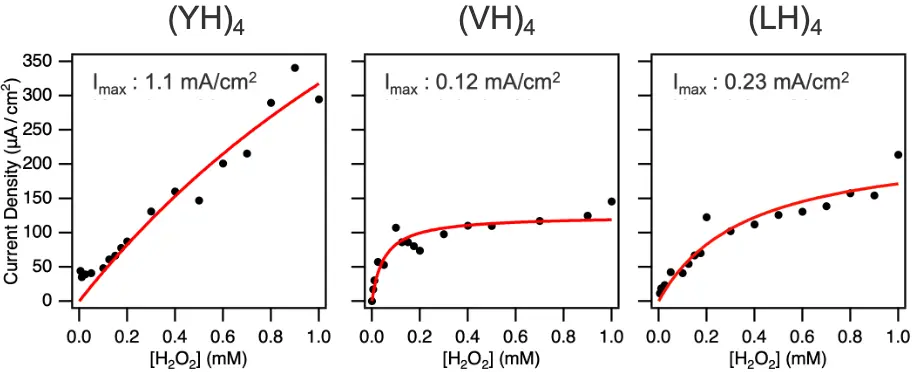
Figure 4. Current density at −0.8 V as a function of H2O2 concentration for each peptide, with fitting curves shown as red solid lines. Imax represent the maximal current density. ©2025 American Chemical Society
“This research highlights the potential of simple peptide designs to create artificial enzymes with robust and durable catalytic interfaces for electrochemical applications,” conclude the researchers in their report. “Furthermore, the peptides’ ability to self-assemble on two-dimensional materials makes them promising candidates for biosensing applications.”
- Glossary
Atomic force microscopy
This imaging technique uses a nanosized tip at the end of a cantilever that is scanned over a sample. It can be used to determine the topography of a sample surface from the change in the strength of forces between the tip and the sample with distance, and the resulting deflection of the cantilever. It was first developed in the 1980s but a number of modifications have augmented the functionality of the technique since. It is better suited to imaging biological samples than the scanning tunnelling microscope developed that had been developed because it does not require a conducting sample
In the 2000s Toshio Ando at Kanazawa University was able to improve the scanning speed to such an extent that moving images could be captured. This allowed people to use the technique to visualize molecular processes for the first time.
Catalysts
Catalysts influence the rate of a reaction without actually being used up in the reaction themselves. They can speed up the rate of all kinds of reactions, including the redox chemistry in the reduction of H2O2, and are prized in industry for improving yield and the profitability of processes.
Redox chemistry describes a host of reactions that involve the gaining (reduction) and losing (oxidation) of electrons by ions in the reaction. It sometimes manifests as the gaining of hydrogen (reduction) or oxygen (oxidation). The generation of water and oxygen from H2O2 is an example of a redox reaction where H2O2 is reduced to H2O and O2
Porphyrin
Porphyrin is an organic compound made up of a ring of four substituted “pyrrole” ring molecules strung together with methine bridges (=CH-). Pyrrole is a cyclic molecule with the formula C4H4NH but in porphyrin other groups may be substituted in. An important porphyrin for living organisms is heme, which carries oxygen in the blood. Chlorophyl is also a porphyrin derivative.
Hemin
Hemin is an iron-containing porphyrin found in the blood. It is the catalytic centre for many different proteins including cytochromes, peroxidases, myoglobins and hemoglobin. The iron in hemin is ferric, that is, it is in the +3 oxidation state (Fe3+).
π−π interactions
In aromatic molecules atoms are bound in the ring by π bonds, a type of covalent bond that takes its name from the shape of the electron orbital which forms lobes on either side of the atoms. π−π stacking describes the non-covalent interactions when these rings stack on each other.
- Reference
| Authors: | Marie Sugiyama, Ayhan Yurtsever, Nina Uenodan, Yuta Nabae, Takeshi Fukuma, and Yuhei Hayamizu |
|---|---|
| Title: | Hierarchical Assembly of Hemin-Peptide Catalytic Systems on Graphite Surfaces |
| Journal: | ACS NANO 2025 |
| DOI: |
10.1021/acsnano.4c15373 |
| Funding: | Y.H. acknowledges support from the Precise Measurement Technology Promotion Foundation (PMTP-F), JSPS KAKENHI Grants 20H02564, 20H03593, 22H05408 and 24H01124, and JST CREST Grant Number JPMJCR24A4, Japan. T.F. acknowledges support from the World Premier International Research Center Initiative (WPI), MEXT, Japan, and JSPS KAKENHI Grant Number 21H05251. |
Related articles
- Electronic Nose: Sensing the Odor Molecules on Graphene Surface Layered with Self-Assembled Peptides | Former Tokyo Tech
- Controlling the interface between bio and nano | Former Tokyo Tech
- New protein bridges chemical divide for 'seamless' bioelectronics devices | Former TMDU
- Yuhei Hayamizu | Researcher Finder - Science Tokyo STAR Search
- Hayamizu Lab
- Materials Science and Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Human Centered Science and Biomedical Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Materials Science and Engineering Undergraduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- School of Materials and Chemical Technology | Science Tokyo organization | About Science Tokyo
- WPI Nano Life Science Institute, Kanazawa University
Further information
Associate Professor Yuhei Hayamizu
School of Materials and Chemical Technology,
Institute of Science Tokyo
E-mail : hayamizu@mct.isct.ac.jp