Materials Science and Engineering News
Exploring Interface Phenomena for More Durable and Effective Nickel–Tungsten Alloys
The insights into the formation of various phases, including intermetallic compounds, at the interface between nickel (Ni) and tungsten (W) by researchers from Tokyo Tech can lead to the development of advanced high-temperature Ni—W coatings. Their study sheds light on the formation of intercrystallite regions and Kirkendall voids, which can be leveraged to improve the durability and effectiveness of the alloys.
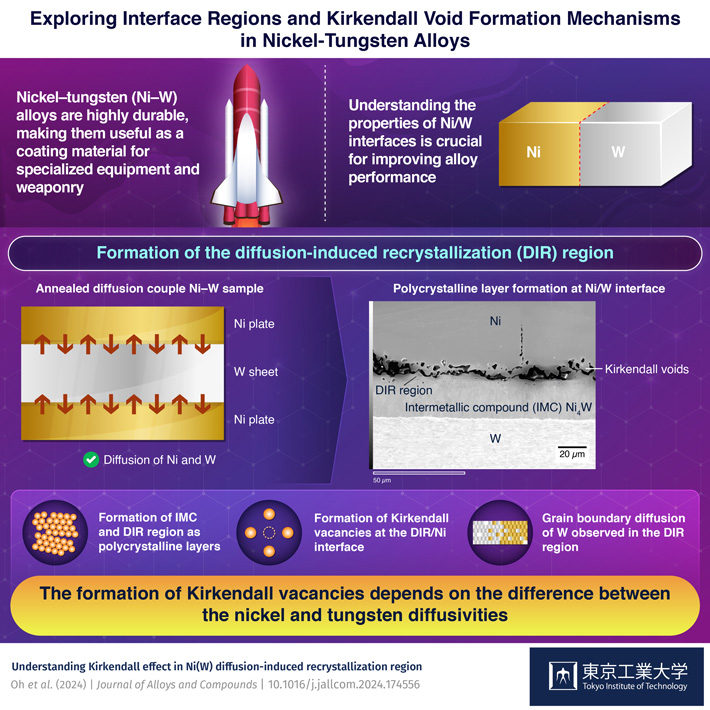
Alloying is a key process in creating new materials. By combining metals with desirable traits, scientists can produce alloys with suitable properties. For example, stainless steel, formed by combining iron with chromium, nickel, and other elements in smaller quantities is highly resistant to corrosion. A class of alloy of particular interest for military applications is the nickel–tungsten alloys (Ni—W). These alloys possess high durability, making them useful as coatings. As Ni and W have different properties, their joining interface forms unique layers where intermetallic compounds (IMCs) and diffusion induced recrystallization (DIR) regions are formed through processes such as diffusion and interfacial reactions. These regions exhibit significantly different mechanical, thermal, and chemical behaviors compared to the rest of the alloy. Therefore, understanding the properties of these interfaces is an important aspect of designing alloys with suitable properties.
Now, researchers led by Assistant Professor Minho Oh from Tokyo Institute of Technology and including Professor Hee-Soo Kim, currently at Chosun University, South Korea, have revealed how various phases, including IMCs, are formed within a Ni—W alloy. Their findings published in the Journal of Alloys and Compounds on April 21, 2024![]() , can prove valuable in developing Ni—W alloys that last longer and are more effective as coatings.
, can prove valuable in developing Ni—W alloys that last longer and are more effective as coatings.
"Insights from studies of IMCs and intermediate layers formed by diffusion at the Ni/W interface have the potential to significantly improve the effectiveness and longevity of important materials in various fields," reiterates Oh.
To examine the Ni/W interface, the researchers sandwiched a W sheet between two Ni plates. They then heated the sample at 1123 K for 112 hours to encourage diffusion, followed by annealing at the same temperature for 234.15 hours. Subsequently, the researchers analyzed the morphology and chemical compositions of the interface using experimental techniques. They analyzed the concentrations of Ni and W in each phase of the material's cross-section, as well as the grain sizes of the regions formed at the interface.
Additionally, the researchers developed a diffusion model that accounted for the diffusion rates of Ni and W in both the bulk metal and different interface regions to explain the formation of these interface regions.
Their analysis revealed that the interdiffusion of Ni and W results in an IMC layer of Ni4W, which grows bidirectionally towards Ni and W plates (Figure 1). The W atoms continue to move into the Ni matrix, forming a diffusion-induced recrystallized region (DIR) between the Ni matrix and the IMC layer (Figure 2). Notably, both the Ni4W IMC and the DIR region exhibit a polycrystalline structure.
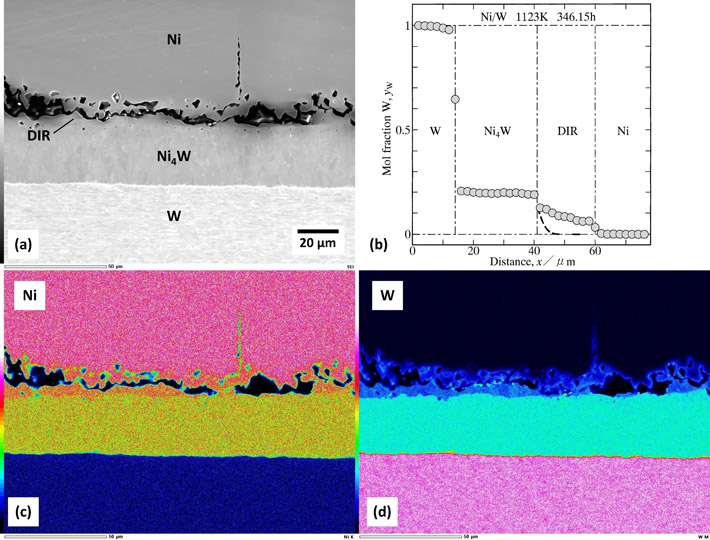
Figure 1. Kirkendall effect in Ni—W alloy system
-
The interdiffusion of Ni and W results in the formation of Ni4W as an intermetallic layer. At the interface between the Ni4W layer and Ni phase, Kirkendall voids are observed.
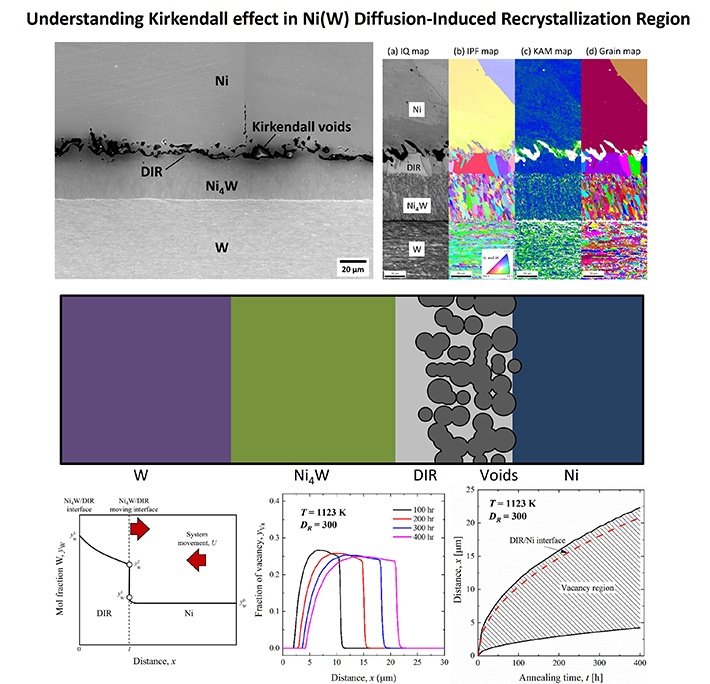
Figure 2. Understanding the Kirkendall effect in Ni (W) diffusion-induced recrystallization (DIC) region
-
In the DIC region, W atoms diffuse into the Ni matrix via grain boundary diffusion.
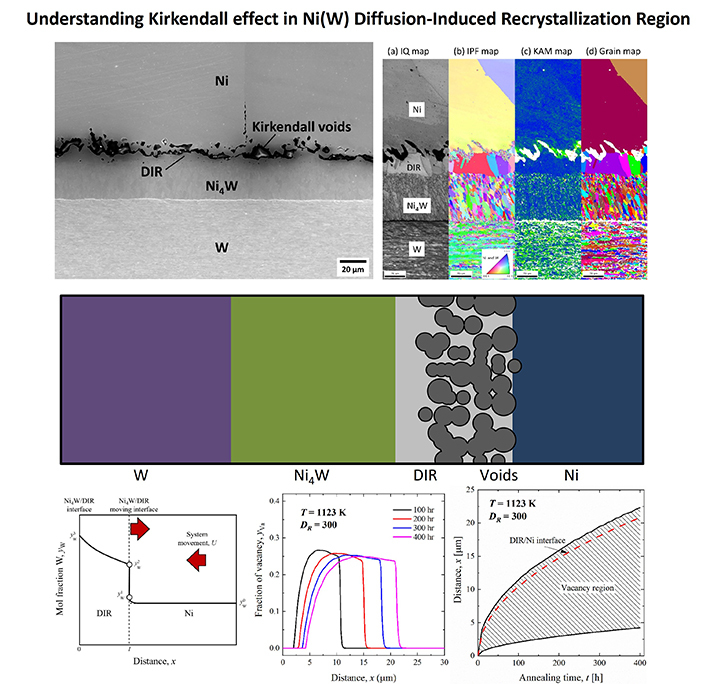
Figure 2. Understanding the Kirkendall effect in Ni (W) diffusion-induced recrystallization (DIC) region
In the DIC region, W atoms diffuse into the Ni matrix via grain boundary diffusion.
The DIR region is not an individual phase but a solid-solution region within the Ni phase.. It is characterized by the presence of elongated columnar-shaped grains that facilitate grain boundary diffusion of W atoms. In the DIR region, the imbalance in the diffusion rates of Ni and W causes irregular-shaped voids known as Kirkendall voids to form near the interface between Ni and DIR in the DIR region. Notably, the interfaces consisting of the DIR region, IMC, and voids influence the strength and thermal properties of the material.
"These findings not only advance our comprehension of the DIR region resulting from IMC formation and diffusion at the Ni/W interface but also offer crucial insights into the phenomenon of Kirkendall void generation and the mechanism of defect formation within the DIR region of the metal system," remarks Oh. "This integrated approach enhances our understanding of thermodynamics and kinetics in the Ni—W diffusion couple, advancing knowledge crucial for high-temperature materials science."
- Reference
| Authors : | Minho Oh1,*, Hee-Soo Kim1,2, Equo Kobayashi1, and Masanori Kajihara1 |
|---|---|
| Title : | Understanding Kirkendall effect in Ni(W) diffusion-induced recrystallization region |
| Journal : | Journal of Alloys and Compounds |
| DOI : | 10.1016/j.jallcom.2024.174556 |
| Affiliations : | 1Department of Materials Science and Engineering, Tokyo Institute of Technology, Japan 2Department of Materials Science and Engineering, Chosun University, South Korea |
|
* Corresponding author's email: o.m.aa@m.titech.ac.jp |
|
- Mitigating corrosion by liquid tin could lead to better cooling in fusion reactors | Tokyo Tech News
- Research video: Liquid Metal, Shaping the World | Tokyo Tech News
- Sustainable construction using eco-friendly concrete: Press webinar with Assoc. Prof. Masatoshi Kondo | Tokyo Tech News
- Minho O | Researcher Finder - Tokyo Tech STAR Search
- Equo Kobayashi | Researcher Finder - Tokyo Tech STAR Search
- Minho O | Google scholar
- Equo Kobayashi Laboratory | School of Materials and Chemical Technology Laboratory Search Site
- Materials Science and Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Human Centered Science and Biomedical Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Materials Science and Engineering Undergraduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Chosun University
- Latest Research News
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Assistant Professor Minho Oh
School of Materials and Chemical Technology, Tokyo Institute of Technology
Email o.m.aa@m.titech.ac.jp