Materials Science and Engineering News
Durable, Inexpensive Catalyst Reduces Carbon Footprint of Ammonia Production
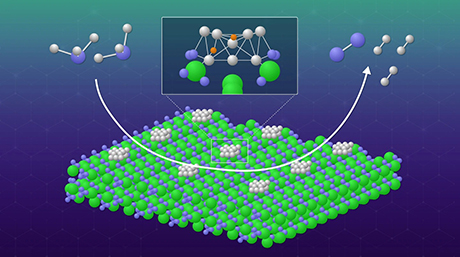
To reduce the energy requirements of the Haber-Bosch process, which converts nitrogen and hydrogen to ammonia, researchers from Tokyo Tech have developed a metal nitride catalyst containing an active metal (Ni) on a lanthanum nitride support that is stable in presence of moisture. Since the catalyst doesn't contain ruthenium, it presents an inexpensive option for reducing the carbon footprint of ammonia production.

The Haber-Bosch process, which is commonly used to synthesize ammonia (NH3)–the foundation for synthetic nitrogen fertilizers–by combining hydrogen (H2) and nitrogen (N2) over catalysts at high pressures and temperatures, is one of the most important scientific discoveries that has helped improve crop yields and increase food production globally.
However, the process requires high fossil fuel energy inputs due to its requirements of high temperatures and pressure. Hydrogen used for this process is produced from natural gas (mainly methane). This hydrogen-producing process is energy-consuming and accompanies huge emission of carbon dioxide. To overcome these issues, various catalysts have been developed to allow the reaction to proceed under milder conditions using hydrogen produced by water electrolysis via renewable energy. Among them are nitride-based catalysts that contain active metal nanoparticles like nickel and cobalt (Ni, Co) loaded on lanthanum nitride (LaN) supports. In these catalysts, both the support and the active metal are involved in the production of NH3. The active metal splits the H2 while the LaN support contains nitrogen vacancies and nitrogen atoms in its crystal structure that adsorb and activate nitrogen (N2). While these catalysts are inexpensive (since they avoid using ruthenium, which is costly), their catalytic performance is degraded in the presence of moisture, with the LaN support transforming into lanthanum hydroxide (La(OH)3).
Now, in a new study published in Angewandte Chemie![]() , researchers from China and Japan led by Professor Hideo Hosono from the Tokyo Institute of Technology (Tokyo Tech), Japan, have developed a chemically stable catalyst that is stable in the presence of moisture. Taking inspiration from stable rare-earth compounds containing chemical bonds between a rare-earth metal (in this case, La) and a metal, they incorporated aluminum atoms into the LaN structure and synthesized a chemically stable La3AlN support containing La-Al bonds that prevent lanthanum atoms from reacting with moisture.
, researchers from China and Japan led by Professor Hideo Hosono from the Tokyo Institute of Technology (Tokyo Tech), Japan, have developed a chemically stable catalyst that is stable in the presence of moisture. Taking inspiration from stable rare-earth compounds containing chemical bonds between a rare-earth metal (in this case, La) and a metal, they incorporated aluminum atoms into the LaN structure and synthesized a chemically stable La3AlN support containing La-Al bonds that prevent lanthanum atoms from reacting with moisture.
The La-Al-N support along with the active metals, such as nickel and cobalt (Ni, Co), was able to produce NH3 at rates similar to that with conventional metal nitride catalysts and could maintain a stable production when fed with nitrogen gas-containing moisture. "The Ni- or Co-loaded La-Al-N catalysts showed no distinct degradation following exposure to 3.5% moisture," says Prof. Hosono.
While the Al atoms stabilized the support, the lattice nitrogen and nitrogen defects present in the doped support enabled the synthesis of ammonia in a manner similar to the conventional active metal/rare-earth metal nitride catalysts. "Lattice nitrogen as well as nitrogen vacancy in La-Al-N play a key role in N2 adsorption, with the La-Al-N support and the active metal Ni being responsible for N2 and H2 absorption and activation, respectively," explains Prof. Hosono.
The Haber-Bosch process is an energy-intensive chemical reaction, accounting for about 1% of global annual carbon dioxide emissions. While alternative environmentally friendly approaches for NH3 production are being investigated, introducing inexpensive catalysts could provide immediate benefits by allowing the process to operate under milder conditions.
- Reference
| Authors : | Yangfan Lu[a],[c]*, Tian-Nan Ye[b], Jiang Li[c], Zichuang Li[b], Haotian Guan[a], Masato Sasase[c], Yasuhiro Niwa[d],[e],[f], Hitoshi Abe[d],[e],[f], Qian Li[a],[g], Fushen Pan[a], Masaaki Kitano[c]* and Hideo Hosono[c]* |
|---|---|
| Title of original paper : | Approach to Chemically Durable Nickel and Cobalt Lanthanum-Nitride-Based Catalysts for Ammonia Synthesis |
| Journal : | Angewandte Chemie International Edition |
| DOI : |
10.1002/anie.202211759 |
| Affiliations : |
[a] College of Materials Science and Engineering, National Engineering Research Center for Magnesium Alloys, Chongqing University [b] Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University [c] Materials Research Center for Element Strategy, Tokyo Institute of Technology [d] Institute of Materials Structure Science, High Energy Accelerator Research Organization [e] Department of Materials Structure Science, School of High Energy Accelerator Science, SOKENDAI, The Graduate University for Advanced Studies [f] Graduate School of Science and Engineering, Ibaraki University [g] State Key Laboratory of Advanced Special Steel, Shanghai Key Laboratory of Advanced Ferro-metallurgy, School of Materials Science and Engineering, Shanghai University |
|
* Corresponding author's email: hosono@msl.titech.ac.jp |
|
- Making Metal–Halide Perovskites Useful in Planar Devices Through a New Hybrid Structure | Tokyo Tech News
- Scientists Observe Record High Hydride Ion Conductivity Using Modified Lanthanum Trihydride | Tokyo Tech News
- Breaking Ammonia: A New Catalyst to Generate Hydrogen from Ammonia at Low Temperatures | Tokyo Tech News
- Turning the Heat Down: Catalyzing Ammonia Formation at Lower Temperatures with Ruthenium | Tokyo Tech News
- Having it Both Ways: A Combined Strategy in Catalyst Design for Suzuki Cross-Couplings | Tokyo Tech News
- Filling the Void in Ammonia Synthesis: The Role of Nitrogen Vacancies in Catalysts | Tokyo Tech News
- Running on Empty: New Affordable Catalyst Relies on Nitrogen Vacancies to Produce Ammonia | Tokyo Tech News
- Efficient bottom-up synthesis of new perovskite material for the production of ammonia | Tokyo Tech News
- Highly efficient ammonia synthesis catalyst developed | Tokyo Tech News
- Kitano Lab. (Japanese)
- Hideo Hosono | Researcher Finder - Tokyo Tech STAR Search
- Masaaki Kitano | Researcher Finder - Tokyo Tech STAR Search
- MDX Research Center for Element Strategy (Former Materials Research Center for Element Strategy)
- Laboratory for Materials and Structures
- Institute of Innovative Research (IIR)
- Materials Science and Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Materials Science and Engineering Undergraduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Chongqing University
- Shanghai Jiao Tong University
- High Energy Accelerator Research Organization (KEK)
- Latest Research News
Further Information
Honorary Professor Hideo Hosono
MDX Research Center for Element Strategy,
Tokyo Institute of Technology
Email hosono@msl.titech.ac.jp