Materials Science and Engineering News
A nanoscale lattice of palladium and yttrium makes for a superlative carbon-linking catalyst
A group of materials scientists at Tokyo Institute of Technology has shown that a palladium-based intermetallic electride, Y3Pd2, can improve the efficiency of carbon–carbon cross-coupling reactions. Their findings point the way to a more sustainable world through catalysis.
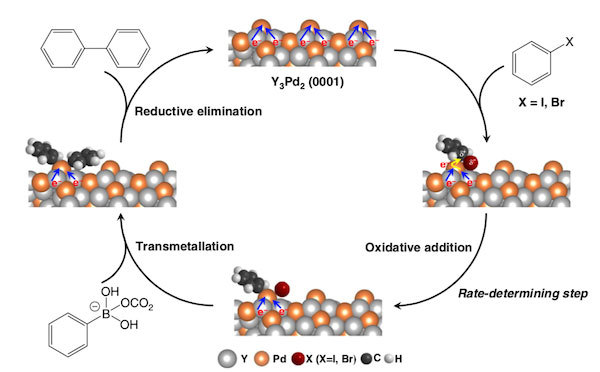
Figure 1. Linking carbon–carbon bonds with Y3Pd2
- Proposed reaction paths for the Suzuki cross-coupling process.
Researchers at Tokyo Institute of Technology (Tokyo Tech) have developed an electride1 material composed of yttrium and palladium (Y3Pd2) as a catalyst for Suzuki cross-coupling reactions. These reactions are among the most widely used for the formation of carbon–carbon bonds in organic and medicinal chemistry.
Y3Pd2 was predicted to be an effective electride based on theoretical calculations, explains Tian-Nan Ye, an assistant professor at Tokyo Tech's Materials Research Center for Element Strategy and first author of the study published in Nature Communications. "In an electride, anionic electrons are trapped in interstitial sites and typically host a strong electron donation effect," he says. "This feature motivated us to apply Y3Pd2 as a Suzuki coupling reaction catalyst as the reaction barrier of the rate-determining step can be suppressed through electron transfer from the electride to the substrates."
In lab tests, the catalytic activity of Y3Pd2 was shown to be ten times higher than that achieved by a pure Pd catalyst, and the activation energy was reduced by 35%.
What makes Y3Pd2 so efficient and stable is the successful incorporation of active Pd atoms in an intermetallic electride lattice. "The stabilized Pd active sites in our crystalline lattice solve the problems of aggregation and leaching that have commonly occurred in other systems reported so far," says Ye. "This makes our catalyst extremely robust and stable for long-term usage, without deactivation."
The reusability of the catalyst (up to 20 cycles) and the relative ease with which Pd atoms can be recovered represents an important step to achieving greater sustainability in the chemical industry.
The idea of combining yttrium and palladium was sparked by the work of Jens Kehlet Nørskov, now at Stanford University, says Ye. In 2009, Nørskov and co-workers published groundbreaking findings on catalysts made of platinum alloyed with early transition metals, including yttrium. Since then, many groups have been investigating new combinations of intermetallic compounds (consisting of a rare earth metal and an active transition metal), with the goal of developing much more efficient catalysts for the chemical industry.
Through a series of calculations and experimental studies, Ye and his team showed that Y3Pd2 has a strong electron-donating effect associated with a low work function and high carrier density — features that enable the catalyst to work at a much lower activation energy than that of a pure Pd catalyst.
One remaining challenge is the relatively low surface area of Y3Pd2. To tackle this issue, the team used a pulverizing technique called ball-milling2 and compared catalytic activity using different solvents such as heptane and ethanol. In all of the samples investigated so far, the team found that the Suzuki coupling reaction rate increased in proportion to the increase in surface area. These initial results are "very promising," says Ye, suggesting that "catalytic performance could be improved through further nanocrystallization."
A crystalline ionic material in which electrons act as anions.
Here referring to a method of grinding materials to a fine powder without disrupting their catalytic ability.
- Reference
| Title : | Palladium-bearing intermetallic electride as an efficient and stable catalyst for Suzuki cross-coupling reactions |
|---|---|
| Authors : | Tian-Nan Ye1,6, Yangfan Lu1,6, Zewen Xiao2,6, Jiang Li1, Takuya Nakao3, Hitoshi Abe4,5, Yasuhiro Niwa4, Masaaki Kitano1, Tomofumi Tada1 & Hideo Hosono1 |
| Journal : | Nature Communications (2019) |
| DOI : | 10.1038/s41467-019-13679-0 |
| Affiliations : |
1Materials Research Center for Element Strategy, Tokyo Institute of Technology 2Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology 3Laboratory for Materials and Structures, Tokyo Institute of Technology 4High Energy Accelerator Research Organization, KEK 5Department of Materials Structure Science, School of High Energy Accelerator Science, SOKENDAI, The Graduate University for Advanced Studies 6These authors contributed equally: Tian-Nan Ye, Yangfan Lu, Zewen Xiao. |
- Efficient bottom-up synthesis of new perovskite material for the production of ammonia | Tokyo Tech News
- New design strategy brightens up the future of perovskite-based light-emitting diodes | Tokyo Tech News
- Finding a needle in a haystack: Discovery of Ti2InB2 for synthesizing layered TiB | Tokyo Tech News
- Lighting it up: A new non-toxic, cheap, and stable blue photoluminescent material | Tokyo Tech News
- Hideo Hosono's story of IGZO TFT development features in Nature Electronics | Tokyo Tech News
- High Performance Nitride Semiconductor for Environmentally Friendly Photovoltaics | Tokyo Tech News
- Beyond the limits of conventional electronics: stable organic molecular nanowires | Tokyo Tech News
- Highly efficient ammonia synthesis catalyst developed | Tokyo Tech News
- Hideo Hosono - Manipulating electrons well to elicit the potential of materials, Part 1 | Research Stories | Research
- Hideo Hosono - Manipulating electrons well to elicit the potential of materials, Part 2 | Research Stories | Research
- Hideo Hosono wins Von Hippel Award from Materials Research Society | Tokyo Tech News
- Researcher Profile | Tokyo Tech STAR Search - Hideo Hosono
- Researcher Profile | Tokyo Tech STAR Search - Masaaki Kitano
- Materials Research Center for Element Strategy
- Alloys of platinum and early transition metals as oxygen reduction electrocatalysts | Nature Chemistry
- Latest Research News
Further Information
Assistant Professor Tian-Nan Ye
Materials Research Center for Element Strategy,
Tokyo Institute of Technology
Email ytn2015@mces.titech.ac.jp
Honorary Professor Hideo Hosono
Materials Research Center for Element Strategy,
Tokyo Institute of Technology
Email hosono@mces.titech.ac.jp
Tel +81-45-924-5009