Materials Science and Engineering News
Developing a Superbase-Comparable BaTiO₃-xNy Oxynitride Catalyst
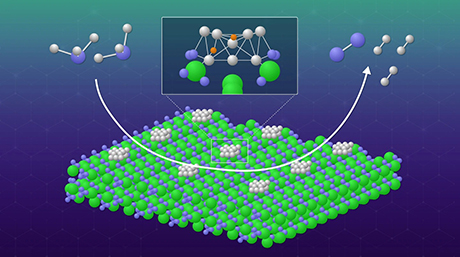
A hexagonal BaTiO3−xNy oxynitride catalyst with a basicity comparable to superbases has been developed by researchers from Tokyo Tech. The high basicity was attributed to the combined effect of substituting nitride ions and oxygen vacancies into BaTiO3. These modifications changed the electronic structure of the catalyst and improved its ability to accept protons, even from highly basic reactants.
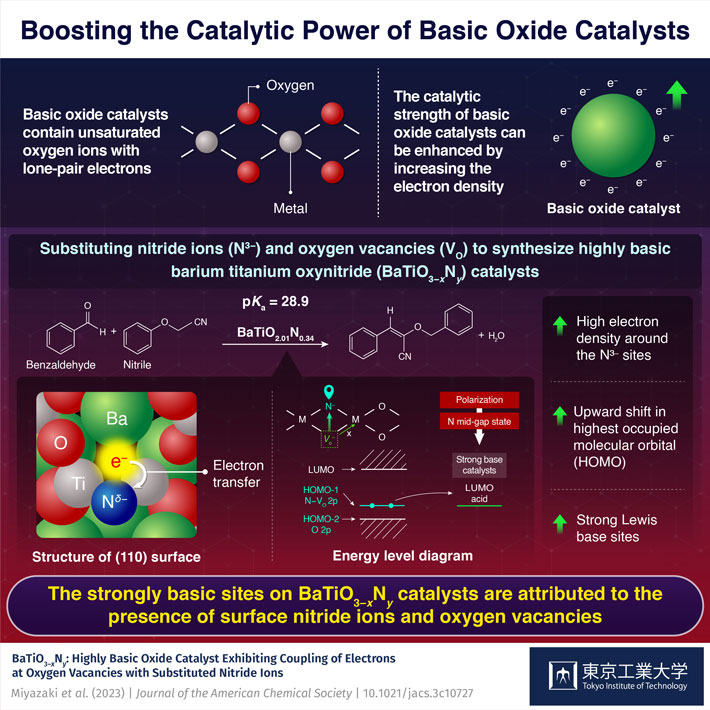
Basic oxide catalysts contain oxygen ions with unpaired electrons that can be shared with other species to facilitate a chemical reaction. These catalysts are widely used in the synthesis of chemicals, pharmaceuticals, and petrochemicals. There have been efforts to improve the catalytic power of these catalysts by improving their basicity or the ability to donate electrons or accept hydrogen ions. Various strategies include doping the catalyst with highly electronegative cations such as alkali metals, substituting oxide ions with anions of different valences, like hydride (H-) or nitride (N3-) ions, or increasing the electron density in the catalyst by introducing oxygen vacancies next to oxide anions.
In a recent study, a team of researchers, led by Assistant Professor Masayoshi Miyazaki and including corresponding authors, Professor Hideo Hosono and Professor Masaaki Kitano, all from Tokyo Institute of Technology (Tokyo Tech), has now developed a hexagonal BaTiO3−xNy oxynitride catalyst with basicity comparable to that of superbases. They achieved this by substituting nitride ions and oxygen vacancies into face-sharing Ti2O9 dimer sites in BaTiO3−x. Their study, published in the Journal of the American Chemical Society![]() on 20 November 2023, lays the groundwork for the development of highly basic catalysts.
on 20 November 2023, lays the groundwork for the development of highly basic catalysts.
The substitution of oxygen ions with nitride ions changes the electronic structure of the catalyst and shifts the energy level of the highest occupied molecular orbitals (HOMO) upward. HOMO represents the highest energy level at which electrons are present in a molecular orbital and the upward shift makes it more favorable for electrons to be donated to a reactant's lowest unoccupied molecular orbital (LUMO). Additionally, the introduction of oxygen vacancies adjacent to the doped nitride ions increases the electron density, further raising the HOMO energy level, resulting in a highly basic catalyst with a high tendency to donate electrons.
Due to this synergetic effect, the developed oxynitride was more basic compared to materials like BaTaO2N and LaTiO2N, which do not contain oxygen vacancies. "This improved basicity stems from the coupling of substituted nitride ions to electrons at oxygen vacancies," explains Dr. Miyazaki.
The strong basicity of the oxynitride catalyst facilitated Knoevenagel condensation reactions. In these reactions, a basic catalyst accepts a proton (hydrogen ion) from the methylene group, leading to the formation of a C–C bond between the carbonyl and methylene groups.
On reacting nitriles (containing the methylene group) with benzaldehyde (representing the carbonyl group), the researchers noted that the oxynitride catalyst BaTiO2.01N0.34 could accept protons from highly basic nitrile reactants with pKa value (the negative logarithm of the acid dissociation constant (Ka) of a compound in water; a high pKa value signifies a weak acid or a strong base) as high as 23.8 and 28.9. In this regard, the catalyst's ability to accept hydrogen ions from highly basic nitrile reactants indicates a basic strength comparable to that of superbases, which have pKa values around 26.
In addition to its highly basic nature, the oxynitride catalyst was stable, undergoing no changes in the structure or electronic state after the reaction. Moreover, the catalyst maintained its catalytic activity even after repeated use, making it suitable for practical applications.
Overall, the method presented in this study to improve the basicity paves the way for the development of highly basic catalysts for various chemical processes. "The synthesis of more highly basic catalysts will require the combination of surface anion species and vacancies," concludes Dr. Miyazaki.
- Reference
| Authors : | Masayoshi Miyazaki1, Hiroshi Saito1, Kiya Ogasawara1, Masaaki Kitano1,*, and Hideo Hosono1,2,* |
|---|---|
| Title : | BaTiO3–xNy: Highly Basic Oxide Catalyst Exhibiting Coupling of Electrons at Oxygen Vacancies with Substituted Nitride Ions |
| Journal : | Journal of the American Chemical Society |
| DOI : |
10.1021/jacs.3c10727 |
| Affiliations : |
1 MDX Research Center for Element Strategy, International Research Frontiers Initiative, Tokyo Institute of Technology, Japan 2 Wpi-MANA, National Institute for Materials Science, Japan |
|
* Corresponding authors' emails: kitano.m.aa@m.titech.ac.jp (Masaaki Kitano); hosono@mces.titech.ac.jp (Hideo Hosono) |
|
- Water-Durable Perovskite-Oxynitride Supported Nickel Catalysts for Ammonia Decomposition | Tokyo Tech News
- Record Ammonia Production Achieved with Inexpensive Cobalt Catalyst at Low Temperatures | Tokyo Tech News
- Opening a New Frontier: PdMo Intermetallic Catalyst for Promoting CO2 Utilization | Tokyo Tech News
- Efficient bottom-up synthesis of new perovskite material for the production of ammonia | Tokyo Tech News
- Turning the Heat Down: Catalyzing Ammonia Formation at Lower Temperatures with Ruthenium | Tokyo Tech News
- Hideo Hosono - Manipulating electrons well to elicit the potential of materials, Part 1 | Research Stories | Research
- Hideo Hosono - Manipulating electrons well to elicit the potential of materials, Part 2 | Research Stories | Research
- Masayoshi Miyazaki | Researcher Finder - Tokyo Tech STAR Search
- Masaaki Kitano | Researcher Finder - Tokyo Tech STAR Search
- Hideo Hosono | Researcher Finder - Tokyo Tech STAR Search
- Kitano Laboratory (Japanese)
- MDX Research Center for Element Strategy (MDXES)
- International Research Frontiers Initiative
- Materials Science and Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Materials Science and Engineering Undergraduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Latest Research News
Further Information
Assistant Professor Masayoshi Miyazaki
MDX Research Center for Element Strategy, International Research Frontiers Initiative, Tokyo Institute of Technology
Email miyazaki.m.ac@m.titech.ac.jp
Professor Masaaki Kitano
MDX Research Center for Element Strategy, International Research Frontiers Initiative, Tokyo Institute of Technology
Email : kitano.m.aa@m.titech.ac.jp
Professor Hideo Hosono
MDX Research Center for Element Strategy, International Research Frontiers Initiative, Tokyo Institute of Technology
Email hosono@mces.titech.ac.jp