Materials Science and Engineering News
Dethroning Electrocatalysts for Hydrogen Production with Inexpensive Alternative Material
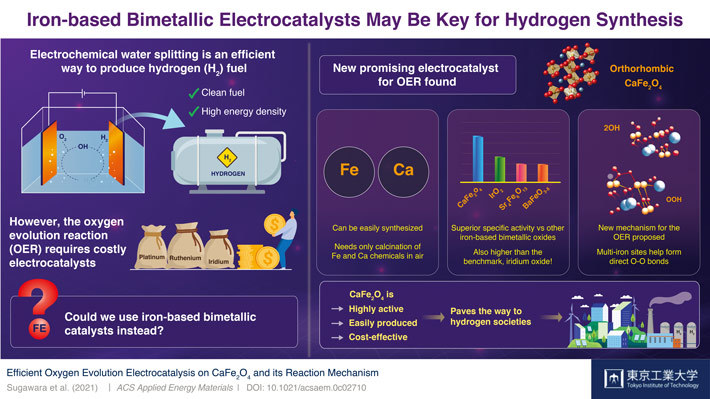
Scientists at Tokyo Institute of Technology (Tokyo Tech) discover a new electrocatalyst for the oxygen evolution reaction (OER) in electrochemical water splitting: CaFe2O4. This inexpensive, non-toxic, and easy-to-synthesize material outperforms other bimetallic OER electrocatalysts and even surpasses the benchmark set by iridium oxide, paving the way to accessible hydrogen generation for upcoming hydrogen societies.
Today, we can say without a shadow of doubt that an alternative to fossil fuels is needed. Fossil fuels are not only non-renewable sources of energy but also among the leading causes of global warming and air pollution. Thus, many scientists worldwide have their hopes placed on what they regard as the fuel of tomorrow: hydrogen (H2). Although H2 is a clean fuel with incredibly high energy density, efficiently generating large amounts of it remains a difficult technical challenge.
Water splitting—the breaking of water molecules—is among the most explored methods to produce H2. While there are many ways to go about it, the best-performing water splitting techniques involve electrocatalysts made from expensive metals, such as platinum, ruthenium, and iridium. The problem lies in that known electrocatalysts made from abundant metals are rather ineffective at the oxygen evolution reaction (OER), the most challenging aspect of the water-splitting process.
In a recent study published in ACS Applied Energy Materials![]() , a team of scientists at Tokyo Institute of Technology, Japan, found a remarkable electrocatalyst candidate for cost-effective water splitting: calcium iron oxide (CaFe2O4). Whereas iron (Fe) oxides are mediocre at the OER, previous studies had noted that combining it with other metals could boost their performance to actually useful levels. However, as Assistant Professor and lead author Dr Yuuki Sugawara comments, no one had focused on CaFe2O4 as a potential OER electrocatalyst. “We wanted to unveil the potential of CaFe2O4 and elucidate, through comparisons with other iron-based bimetallic oxides, crucial factors that promote its OER activity,” he explains.
, a team of scientists at Tokyo Institute of Technology, Japan, found a remarkable electrocatalyst candidate for cost-effective water splitting: calcium iron oxide (CaFe2O4). Whereas iron (Fe) oxides are mediocre at the OER, previous studies had noted that combining it with other metals could boost their performance to actually useful levels. However, as Assistant Professor and lead author Dr Yuuki Sugawara comments, no one had focused on CaFe2O4 as a potential OER electrocatalyst. “We wanted to unveil the potential of CaFe2O4 and elucidate, through comparisons with other iron-based bimetallic oxides, crucial factors that promote its OER activity,” he explains.
To this end, the team tested six kinds of iron-based oxides, including CaFe2O4. They soon found that the OER performance of CaFe2O4 was vastly greater than that of other bimetallic electrocatalysts and even higher than that of iridium oxide, a widely accepted benchmark. Additionally, they tested the durability of this promising material and found that it was remarkably stable; no significant structural nor compositional changes were seen after measurement cycles, and the performance of the CaFe2O4 electrode in the electrochemical cell remained high.
Eager to understand the reason behind the exceptional capabilities of this unexplored electrocatalyst, the scientists carried out calculations using density functional theory and discovered an unconventional catalytic mechanism. It appears that CaFe2O4 offers an energetically favorable pathway for the formation of oxygen bonds, which is a limiting step in the OER. Although more theoretical calculations and experiments will be needed to be sure, the results indicate that the close distance between multiple iron sites plays a key role.
The newly discovered OER electrocatalyst could certainly be a game changer, as Dr Sugawara remarks, “CaFe2O4 has many advantages, from its easy and cost-effective synthesis to its environmental friendliness. We expect it will be a promising OER electrocatalyst for water splitting and that it will open up a new avenue for the development of energy conversion devices.” In addition, the new OER boosting mechanism found in CaFe2O4 could lead to the engineering of other useful catalysts. Let us hope these findings help pave the way to the much-needed hydrogen society of tomorrow!
- Reference
| Authors : | Yuuki Sugawara1, Keigo Kamata2,3*, Atsushi Ishikawa4,3, Yoshitaka Tateyama4,5, and Takeo Yamaguchi1* |
|---|---|
| Title of original paper : | Efficient Oxygen Evolution Electrocatalysis on CaFe2O4 and its Reaction Mechanism |
| Journal : | ACS Applied Energy Materials |
| DOI : | 10.1021/acsaem.0c02710 |
| Affiliations : |
1 Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology 2 Laboratory for Materials and Structures, Institute of Innovative Research, Tokyo Institute of Technology 3 Precursory Research for Embryonic Science and Technology (PRESTO) 4 Center for Green Research on Energy and Environmental Materials (GREEN), National Institute for Materials Science (NIMS) 5 International Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS) |
* Corresponding authors' emails: kamata.k.ac@m.titech.ac.jp, yamag@res.titech.ac.jp
- Yamaguchi Tamaki Laboratory
- Hara & Kamata Laboratory
- Researcher Profile | Tokyo Tech STAR Search - Yuuki Sugawara
- Researcher Profile | Tokyo Tech STAR Search - Takeo Yamaguchi
- Researcher Profile | Tokyo Tech STAR Search - Keigo Kamata
- Laboratory for Chemistry and Life Science, Institute of Innovative Research
- Laboratory for Materials & Structures, Institute of Innovative Research
- Institute of Innovative Research (IIR)
- Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- Materials Science and Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Energy Science and Engineering Graduate Major|Education|Department of Materials Science and Engineering, School of Materials and Chemical Technology
- National Institute for Materials Science (NIMS)
- Latest Research News
Further Information
Assistant Professor Yuuki Sugawara
Institute of Innovative Research,
Tokyo Institute of Technology