Life Science and Technology News
【Labs spotlight】 Nishiyama and Miura Laboratory
Development of smart nanomedicine based on polymer nanotechnology
The Department has a variety of laboratories for Life Science and Technology, in which cutting-edge innovative research is being undertaken not only in basic science and engineering but also in the areas of medicine, pharmacy, agriculture, and multidisciplinary sciences.
This "Spotlight" series features a laboratory from the Department and introduces you to the laboratory's research projects and outcomes. This time we focus on Nishiyama Laboratory.

Areas of Supervision
Primary/Science and Technology for health Care and Medicine
Secondary/Life Science and Technology, Human Centered Science and Biomedical Engineering
Professor Nobuhiro Nishiyama![]()
| Degree | PhD 2001, The University of Tokyo |
|---|---|
| Areas of Research | DDS, Biomaterials Science |
| Keywords | Polymer, Targeting, Imaging, Nanomedicine |
| Website | Nishiyama Laboratory |
Research interest
Recent advances in nanobiotechnology have led to the development of various biofunctional molecules such as proteins, peptides, nucleic acids, environment/stimuli-responsive materials, imaging molecules, and their application to medical field is expected. However, with these molecules alone, it is difficult to obtain the desired function and effect in vivo, and sometimes the side effects are a serious problem. In the Nishiyama laboratory, we develop nanomedicines based on the platform of fine-tuning synthetic polymers. By integrating various functionalities such as targetability and environment sensitivity, we aim to realize smart diagnosis and therapy in a spatiotemporally controllable manner. We welcome highly motivated students with a passion for developing innovative medicines.
As a recent achievement, we have succeeded in developing a novel cancer cell-binding polymers recognizing the density of glutamine transporters via multivalent interactions (Sci. Rep. 2017) (Fig. 1 (A)). Thus, we are developing various polymers that change their properties in response to various environment/stimuli, aiming at application to nanomedicine. In addition, we are also developing elemental technologies for smart nanomedicines. As a recent achievement, we developed a new cytoplasmic environment-specific linker cleavable in the presence of Glutathione / Glutathione S-transferase (GSH/GST) (ChemMedChem. 2016) (Fig. 1 (B)). We have succeeded in improving the expression efficiency of the polymer-siRNA conjugate using this linker. Regarding diagnostic and therapeutic applications, we have successfully developed nanoparticle MRI contrast agents that increase signal intensity in response to low pH environment in cancer tissues (Nature Nanotech. 2016). Utilizing this system, we succeeded in selective imaging hypoxic region (Hypoxia) in the tumor and to detect small cancer of 1.5 mm with high sensitivity (FIG. 1 (C)). In addition, we are developing nanomedicines that improve the selectivity and enhance the efficacy of therapeutics such as anticancer drugs and nucleic acid drugs. Some of our systems have already been advanced to clinical trials. As a recent achievement, we have developed a polymer conjugate capable of uniformly delivering boron clusters into cancer tissues, and excellent therapeutic effects by boron neutron capture therapy (BNCT) have been confirmed (J. Control. Release 2016) (FIG. 1 (D)).
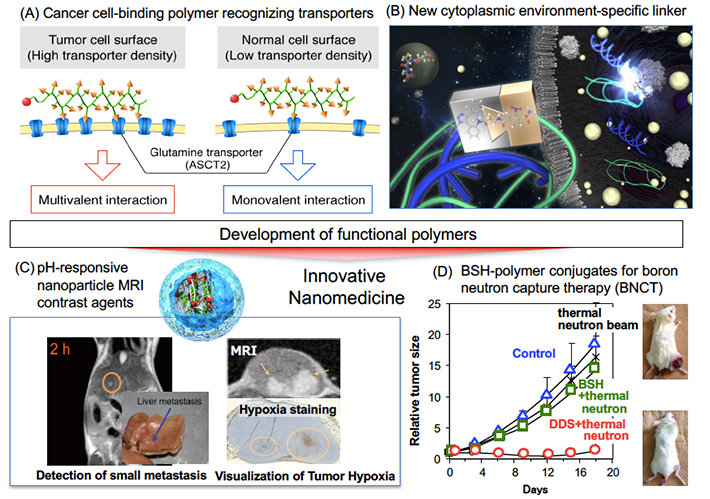
Fig.1 Development of functional polymers and smart diagnostic and therapeutic systems based on polymer nanotechnology
We hope to contribute to realizing innovative diagnostic and therapeutic systems in collaboration with medical institutions and industries, to contribute to the advance in medical care.

Fig.2 Group photo of Nishiyama Lab.
Research findings
Selected publications
- [1] N. Yamada, Y. Honda, H. Takemoto, T. Nomoto, Makoto M., K. Tomoda, M. Konno, H. Ishii, M. Mori, N. Nishiyama*, Engineering tumour cell-binding synthetic polymers with sensing dense transporters associated with aberrant glutamine metabolism. Sci. Rep., in press
- [2] H. Taniguchi, D. Hoshino, C. Moriya, H. Zembutsu, N. Nishiyama, H. Yamamoto, K. Kataoka, K. Imai, Silencing PRDM14 expression by an innovative RNAi therapy inhibits stemness, tumorigenicity, and metastasis of breast cancer. Oncotarget, in press
- [3] P. Mi*, H. Yanagie, N. Dewi, H.-C. Yen, X. Liu, M. Suzuki, Y. Sakurai, K. Ono, H. Takahashi, H. Cabral*, K. Kataoka*, N. Nishiyama*, Block copolymer-boron cluster conjugate for effective boron neutron capture therapy of solid tumors. J. Control. Release, 254 1-9 (2017)
- [4] C. H. Huang, H. Takemoto*, T. Nomoto, K. Tomoda, M. Matsui, N. Nishiyama*, Utility of the 2-nitrobenzenesulfonamide group as a chemical linker for enhanced extracellular stability and cytosolic cleavage in siRNA-conjugated polymer systems. ChemMedChem 12 (1) 19-22 (2017)
- [5] N. F. Che Harun, H. Takemoto*, T. Nomoto, K. Tomoda, M. Matsui, N. Nishiyama*, Artificial control of gene silencing activity based on siRNA conjugation with polymeric molecule having coil-globule transition behavior. Bioconjugate Chem. 27 (9) 1961-1964 (2016)
- [6] H. Hino, M. Kamiya, K. Kitano, K. Mizuno, S. Tanaka, N. Nishiyama, K. Kataoka, Y. Urano*, J. Nakajima*, Rapid cancer fluorescence imaging using a γ-glutamyltranspeptidase-specific probe for primary lung cancer. Transl Oncol. 9 (3) 203-210 (2017)
- [7] M. Iijima, D. Ulkoski, S. Sakuma, D. Matsukuma, N. Nishiyama, H. Otsuka, C. Scholz*, Synthesis of PEGylated poly(amino acid) pentablock copolymers and their self-assembly. Polymer International 65 (10) 1132-1141 (2016)
- [8] P. Mi, D. Kokuryo, H. Cabral, H. Wu, Y. Terada, T. Saga, I. Aoki*, N. Nishiyama*, K. Kataoka*, A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nature Nanotech. 11 (8) 724-730 (2016)
- [9] H. Kinoh, Y. Miura, T. Chida, X. Liu, K. Mizuno, S. Fukushima, Y. Morodomi, N. Nishiyama, H. Cabral*, K. Kataoka*, Nanomedicines eradicating cancer stem-like cells in vivo by pH-triggered intracellular cooperative action of loaded drugs. ACS Nano 10 (6) 5643-5655 (2016)
- [10] Y. Matsumoto, Y. Miyamoto, H. Cabral, Y. Matsumoto, K. Nagasaka, S. Nakagawa*, D. Maeda, T. Yano, K. Oda, K. Kawana, N. Nishiyama, K. Kataoka*, T. Fujii, Enhanced efficacy against cervical carcinomas through polymeric micelles physically incorporating the proteasome inhibitor MG132. Cancer Sci. 107 (6) 773-781 (2016)
- [11] Y. Matsumoto, J. W. Nichols, K. Toh, T. Nomoto, H. Cabral, Y. Miura, R. J. Christie, N. Yamada, T. Ogura, M. R. Kano, Y. Matsumura, N. Nishiyama, T. Yamasoba, Y. -H. Bae*, K. Kataoka*, Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nature Nanotech. 11 (6) 533-538 (2016)
- [12] H. Nishida, Y. Matsumoto, K. Kawana*, R. J. Christie, M. Naito, B. -S. Kim, K. Toh, H. -S. Min, Y. Yi, Y. Matsumoto, H. -J. Kim, K. Miyata*, A. Taguchi, K. Tomio, A. Yamashita, T. Inoue, H. Nakamura, A. Fujimoto, M. Sato, M. Yoshida, K. Adachi, T. Arimoto, O. Wada-Hiraike, K. Oda, T. Nagamatsu, N. Nishiyama, K. Kataoka*, Y. Osuga, T. Fujii, Systemic delivery of siRNA by actively targeted polyion complex micelles for silencing the E6 and E7 human papillomavirus oncogenes. J. Control. Release 231 29-37 (2016)
- [13] M. Wang, Y. Miura, K. Tsuchihashi, K. Miyano, O. Nagano, M. Yoshikawa, A. Tanabe, J. Makino, Y. Mochida, N. Nishiyama, H. Saya, H. Cabral*, K. Kataoka*, Eradication of CD44-variant positive population in head and neck tumors through controlled intracellular navigation of cisplatin-loaded nanomedicines. J. Control. Release 230 26-33 (2016)
- [14] T. Nomoto, S. Fukushima, M. Kumagai, K. Miyazaki, A. Inoue, P. Mi, Y. Maeda, K. Toh, Y. Matsumoto, Y. Morimoto, A. Kishimura, N. Nishiyama*, K. Kataoka*, Calcium phosphate-based organic-inorganic hybrid nanocarriers with pH-responsive on/off switch for photodynamic therapy. Biomater. Sci. 4 (5) 826-838 (2016)
- [15] H. Uchida, K. Itaka, S. Uchida, T. Ishii, T. Suma, K. Miyata, M. Oba, N. Nishiyama, K. Kataoka*, Synthetic polyamines to regulate mRNA translation through the preservative binding of eukaryotic initiation factor 4E to the cap structure. J. Am. Chem. Soc. 138 (5) 1478-1481 (2016)
- [16] Y. Anraku, A. Kishimura*, M. Kamiya, S. Tanaka, T. Nomoto, K. Toh, Y. Matsumoto, S. Fukushima, D. Sueyoshi, M. R. Kano, Y. Urano, N. Nishiyama, K. Kataoka*, Systemically injectable enzyme-loaded polyion complex vesicles as in vivo nanoreactors functioning in tumors. Angew. Chem. Int. Ed. 128 (2) 570-575 (2016)
- [17] P. Mi, N. Dewi, H. Yanagie, D. Kokuryo, M. Suzuki, Y. Sakurai, Y. Li, I. Aoki, K. Ono, H. Takahashi, H. Cabral, N. Nishiyama*, K. Kataoka*, Hybrid calcium phosphate-polymeric micelles incorporating gadolinium chelates for imaging-guided gadolinium neutron capture tumor therapy. ACS Nano 9 (6) 5913-5921 (2015)
- [18] H. Cabral, J. Makino, Y. Matsumoto, P. Mi, H. Wu, T. Nomoto, K. Toh, N. Yamada, Y. Higuchi, S. Konishi, M. R. Kano, H. Nishihara, Y. Miura, N. Nishiyama, K. Kataoka*, Systemic targeting of lymph node metastasis through the blood vascular system by using size-controlled nanocarriers. ACS Nano 9 (5) 4957-4967 (2015)
- [19] H.-C. Yen, H. Cabral, P. Mi, K. Toh, Y. Matsumoto, X. Liu, H. Koori, A. Kim, K. Miyazaki, Y. Miura, N. Nishiyama, K. Kataoka*, Light-induced cytosolic activation of reduction-sensitive camptothecin-loaded polymeric micelles for spatiotemporally controlled in vivo chemotherapy. ACS Nano 8 (11) 11591-11602 (2014)
- [20] T. Nomoto, S. Fukushima, M. Kumagai, K. Machitani, Arnida, Y. Matsumoto, M. Oba, K. Miyata, K. Osada, N. Nishiyama*, K. Kataoka*, Three-layered polyplex micelle as a multifunctional nanocarrier platform for light-induced systemic gene transfer. Nature Commun. 5 3545 (2014)
- [21] H. Chen, L. Xiao, Y. Anraku, P. Mi, X. Liu, H. Cabral, A. Inoue, T. Nomoto, A. Kishimura*, N. Nishiyama*, K. Kataoka*, Polyion complex vesicles for photo-induced intracellular delivery of amphiphilic photosensitizer. J. Am. Chem. Soc. 136 (1) 157-163 (2014)
- [22] Y. Miura, T. Takenaka, K. Toh, S. Wu, H. Nishihara, M. R. Kano, Y. Ino, T. Nomoto, Y. Matsumoto, H. Koyama, H. Cabral, N. Nishiyama*, K. Kataoka*, Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano 7 (10) 8583-8592 (2013)
- [23] H. Cabral, M. Murakami, H. Hojo, Y. Terada, M. R. Kano, U. -I. Chung, N. Nishiyama*, K. Kataoka*, Targeted therapy of spontaneous murine pancreatic tumors by polymeric micelles prolongs survival and prevents peritoneal metastasis. Proc. Natl. Acad. Sci. USA. 110 (28) 11397-11402 (2013)
- [24] H. Takemoto, K. Miyata, S. Hattori, T. Ishii, T. Suma, S. Uchida, N. Nishiyama, K. Kataoka*, Acidic pH-responsive siRNA conjugate for reversible carrier stability and accelerated endosomal escape with reduced IFNα-associated immune response. Angew. Chem. Int. Ed. 52 (24) 6218-6221 (2013)
- [25] H. Cabral, Y. Matsumoto, K. Mizuno, Q. Chen, M. Murakami, M. Kimura, Y. Terada, M.R. Kano, K. Miyazono, M. Uesaka, N. Nishiyama*, K. Kataoka*, Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nature Nanotech. 6 (12) 815-823 (2011)
- [26] M. Murakami, H. Cabral, Y. Matsumoto, S. Wu, M. R. Kano, T. Yamori, N. Nishiyama*, K. Kataoka*, Improving drug potency and efficacy by nanocarrier-mediated subcellular targeting. Science Transl. Med. 3 (64) 64ra2 (2011)
- Research Laboratories and Subjects
- "Nanomachine Contrast Agent" using existing MRI detects minimal cancer tissue with a highly sensitive visualization | Life Science and Technology News
- A nanomachine for "surgery with no incision" developed -- Aiming at outpatient cancer treatment | Tokyo Tech News
- A new nanomachine shows potential for light-selective gene therapy | Tokyo Tech News
- Laboratory for Chemistry and Life Science Institute of Innovative Research
Contact
Professor Nobuhiro Nishiyama
Room 812, R1 building, Suzukakedai campus
Email nishiyama.n.ad@m.titech.ac.jp
*Find more about the lab and the latest activities at the lab site![]() .
.
*May 1, 2025:Some of the content has been updated with the latest information.