Chemical Science and Engineering News
All wired up: New molecular wires for single-molecule electronic devices
Scientists at Tokyo Institute of Technology designed a new type of molecular wire doped with organometallic ruthenium to achieve unprecedentedly higher conductance than earlier molecular wires. The origin of high conductance in these wires is fundamentally different from similar molecular devices and suggests a potential strategy for developing highly conducting "doped" molecular wires.
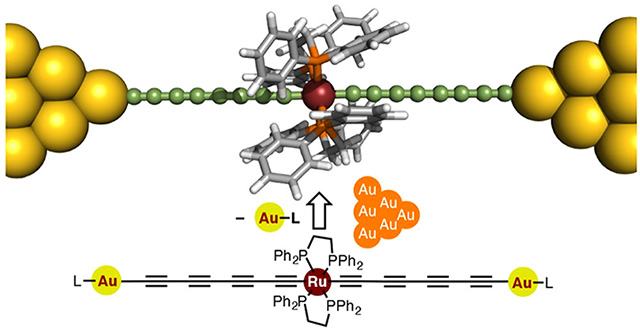
Figure 1. Structure of the proposed metallapolyyne molecular wire
The proposed wire is "doped" with a ruthenium unit that enhances its conductance to unprecedented levels compared with previously reported similar molecular wires.
Since their conception, researchers have tried to shrink electronic devices to unprecedented sizes, even to the point of fabricating them from a few molecules. Molecular wires are one of the building blocks of such minuscule contraptions, and many researchers have been developing strategies to synthesize highly conductive, stable wires from carefully designed molecules.
A team of researchers from Tokyo Institute of Technology, including Yuya Tanaka and Munetaka Akita, designed a novel molecular wire in the form of a metal electrode-molecule-metal electrode (MMM) junction including a polyyne, an organic chain-like molecule, "doped" with a ruthenium-based unit Ru(dppe)2 (Figure 1). The proposed design, featured in the cover of the Journal of the American Chemical Society![]() , is based on engineering the energy levels of the conducting orbitals of the atoms of the wire, considering the characteristics of gold electrodes.
, is based on engineering the energy levels of the conducting orbitals of the atoms of the wire, considering the characteristics of gold electrodes.
Using scanning tunneling microscopy, the team confirmed that the conductance of these molecular wires was equal to or higher than those of previously reported organic molecular wires, including similar wires "doped" with iron units. Motivated by these results, the researchers then went on to investigate the origin of the proposed wire's superior conductance. They found that the observed conducting properties were fundamentally different from previously reported similar MMM junctions and were derived from orbital splitting. In other words, orbital splitting induces changes in the original electron orbitals of the atoms to define a new "hybrid" orbital facilitating electron transfer between the metal electrodes and the wire molecules. According to Tanaka, "such orbital splitting behavior has rarely been reported for any other MMM junction".
Since a narrow gap between the highest (HOMO) and lowest (LUMO) occupied molecular orbitals is a crucial factor for enhancing conductance of molecular wires, the proposed synthesis protocol adopts a new technique to exploit this knowledge, as Tanaka adds "The present study reveals a new strategy to realize molecular wires with an extremely narrow HOMO−LUMO gap via MMM junction formation."
This explanation for the fundamentally different conducting properties of the proposed wires facilitate the strategic development of novel molecular components, which could be the building blocks of future minuscule electronic devices.
Reference
| Authors : | Yuya Tanaka1, Yuya Kato1, Tomofumi Tada2,*, Shintaro Fujii3, Manabu Kiguchi3 and Munetaka Akita1 |
|---|---|
| Title of original paper : | "Doping" of Polyyne with an Organometallic Fragment Leads to Highly Conductive Metallapolyyne Molecular Wire |
| Journal : | Journal of the American Chemical Society |
| DOI : | 10.1021/jacs.8b04484 |
| Affiliations : |
1Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology 2Materials Research Center for Element Strategy, Tokyo Institute of Technology 3Department of Chemistry, School of Science, Tokyo Institute of Technology |
- Across the metal–molecule interface: Observing fluctuations on the single-molecule scale | Tokyo Tech News
- Antiaromatic molecule displays record electrical conductance | Tokyo Tech News
- Bowl-to-bowl inversion: Key to understanding mechano-electronic switches | Tokyo Tech News
- Photocatalytic difluoromethylation of olefins: Simple synthesis of CF2H-containing organic molecules | Tokyo Tech News
- Resolving metal-molecule interfaces at single-molecule junctions | Tokyo Tech News
- Lighting the way to advanced drug design | Tokyo Tech News
- Self-assembled aromatic molecular stacks, towards modular molecular electronic components | Tokyo Tech News
- Spirit of new materials creation│Research Stories|Research
- Akita-Yoshizawa Lab
- Kiguchi and Nishino Laboratory
- Tada Lab
- Researcher Profile | Tokyo Tech STAR Search - Yuya Tanaka
- Researcher Profile | Tokyo Tech STAR Search - Munetaka Akita
- Researcher Profile | Tokyo Tech STAR Search - Tomofumi Tada
- Researcher Profile | Tokyo Tech STAR Search - Shintaro Fujii
- Researcher Profile | Tokyo Tech STAR Search - Manabu Kiguchi
- Department of Chemistry, School of Science
- Department of Materials Science and Engineering, School of Materials and Chemical Technology
- Institute of Innovative Research (IIR)
- Laboratory for Chemistry and Life Science, Institute of Innovative Research
- MCES Tokyo Institute of Technology
- π-System Figuration
- Latest Research News
School of Science —Exploring the Truth and Creating Knowledge—
Information on School of Science inaugurated in April 2016
Further Information
Assistant Professor Yuya Tanaka
Laboratory for Chemistry and Life Sciences, Institute of Innovative Research,
Tokyo Institute of Technology
Email ytanaka@res.titech.ac.jp
Tel +81-45-924-5230
Professor Munetaka Akita
Laboratory for Chemistry and Life Sciences, Institute of Innovative Research,
Tokyo Institute of Technology
Email makita@res.titech.ac.jp
Tel +81-45-924-5230
Contact
Public Relations Section, Tokyo Institute of Technology
Email media@jim.titech.ac.jp
Tel +81-3-5734-2975