Life Science and Technology News
Overcoming the challenges to synthesising iron–sulfur proteins outside the glovebox
Fe–S clusters, which are a part of Fe–S proteins, are found across all forms of life. They play a significant role as biological cofactors—helper molecules that assist these proteins in different biochemical transformations—that are involved in respiration and metabolism. These clusters are of keen research interest since they are considered to be a critical part of evolution. They serve as a link between pre-biotic chemistry (chemical processes that existed prior to the emergence of life forms) and the complex molecular and biological systems we know today. Put simply, they might be one of the primitive catalysts that led to the emergence of life on Earth. Thus, having convenient methods to synthesise Fe–S proteins will hopefully advance our understanding of young Earth biology and help us answer the ultimate question of the origin of life.
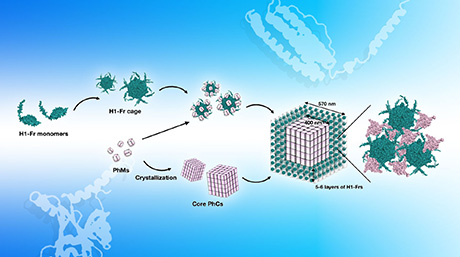
![Figure 1. One-pot cell-free synthesis of mature iron–sulfur proteins This diagram shows the two main steps involved in the proposed synthesis protocol. Step 1 shows the chemical cascade used to create an oxygen-free environment, alongside the PURE system used to synthesise the immature (apo) proteins. Step 2 shows the implementation of the SUF machinery, which adds the [4Fe–4S] cluster to the apo proteins, yielding functional and mature [4Fe–4S] proteins. Credit: Reproduced from Wang and Nishikawa et al. 2023 ACS Synthetic Biology](/bio/eng/news/img/news_230830a_01.jpg)
Figure 1. One-pot cell-free synthesis of mature iron–sulfur proteins
- This diagram shows the two main steps involved in the proposed synthesis protocol. Step 1 shows the chemical cascade used to create an oxygen-free environment, alongside the PURE system used to synthesise the 'immature' (apo) proteins. Step 2 shows the implementation of the SUF machinery, which adds the [4Fe–4S] cluster to the apo proteins, yielding functional and mature [4Fe–4S] proteins. Credit: Reproduced from Wang and Nishikawa et al. 2023 ACS Synthetic Biology
However, despite their prevalence, the synthesis of mature Fe–S proteins outside the cell has proven to be challenging. They not only require intricate cellular machinery for synthesis but also degrade easily upon contact with oxygen due to its reaction with their Fe–S clusters. Therefore, scientists had been forced to follow the complicated route of first producing and extracting an incomplete (or 'apo') protein, followed by its maturation (addition of the Fe–S cofactor) under strictly oxygen-deprived conditions. But what makes this process even harder is the presence of contaminating iron-containing proteins in the final extract.
In a recent study, a team of researchers, including Associate Professors Kosuke Fujishima and Shawn McGlynn from the Earth-Life Science Institute (ELSI), Tokyo Institute of Technology and Assistant Professor Po-Hsiang Wang from the National Central University developed a novel protocol for producing mature [4Fe-4S] proteins in which the Fe–S cluster is placed within a cube-like structure. The team devised a specialised Fe–S assembly protein system pathway that would function in an oxygen-free environment due to the presence of an oxygen-scavenging system to deliver mature Fe–S proteins.
The researchers first sought to assemble what's known as the sulfur formation (SUF) system. In bacteria, this multiprotein system contains all the necessary machinery to produce [4Fe-4S] clusters. It has a higher tolerance for oxygen when compared to the other pathways with similar functions (such as nitrogen fixation and theiron–sulfur cluster system). The research team created a recombinant SUF pathway that consists of six protein subunits with the ability to function in a cell-free environment.
In order to maintain an oxygen-free environment within the test tube, the researchers then introduced a three-enzyme cascade (a set of three enzymatic reactions that occur in a sequence) that serves as an oxygen-scavenging system. While this scavenging system removes oxygen from the environment, it also improves the efficiency of the system. It achieves this by producing reduced flavin adenine dinucleotide (FADH2), an electron-carrier which is needed for the synthesis of Fe-S cluster by SUF system.
Finally, for the synthesis of apo protein, the team adopted a specialised cell-free method that enables the in vitro production of proteins by using reconstituted cell-free protein synthesis known as PURE system. With the addition of genetic material (DNA or mRNA) and the necessary sources of energy, the PURE system essentially acts as an artificial protein factory.
Thus, the researchers combined the PURE system, the O2-scavenging enzyme cascade, and the components of the SUF pathway in a single tube to perform one-pot, cell-free synthesis of two representative [4Fe-4S] proteins: aconitase and thermophilic ferredoxin. Explaining the iterative processes experienced in getting the pieces of the puzzle to fit together, a co-first author PhD student Shota Nishikawa comments, "it was challenging to home-on to the appropriate stoichiometry and substrate/cofactor concentration. Nevertheless, we performed thorough studies to characterise the proper ratio of enzymes needed in the PURE system."
The protocol developed in this study has significant implications for the scientific community. "We have created a novel and convenient method for the synthesis of mature Fe–S proteins which no longer requires a bulky glove box," highlights Fujishima. He claims that their protocol overcomes the traditional challenges of the [4Fe–4S] cluster assembly and O2 sensitivity, which have been major obstacles in the fields of synthetic biology and anaerobic enzymology. By greatly extending the capabilities of the PURE system, the strategy proposed by the researchers could lead to the development of new biotechnologies and a better understanding of the fundamentals of protein synthesis and assembly.
With eyes on the future, the researchers note that this work opens several doors for upcoming studies. Being able to implement in vitro oxygen-free environments could help scientists replicate other types of multiprotein pathways, such as the nitrogen fixation (NIF) andiron–sulfur cluster (ISC) pathways, to synthesise other metal cofactor-bearing enzymes. This, in turn, may lead to the development of new biocatalysts and synthetic cells, with potential applications in environmental remediation, energy production, medicine, and astrobiology.

Figure 2. Pushing the limits of in vitro protein synthesis
- The proposed approach allows for the one-pot production of oxygen sensitive metalloproteins without relying on cell cultures and the glovebox, making it convenient in terms of time and handling. Credit: ELSI
- Reference
| Authors : | Po-Hsiang Wang1,2*, Shota Nishikawa3,4, Shawn Erin McGlynn3,5, and Kosuke Fujishima3,6* |
|---|---|
| Title : | One-Pot De Novo Synthesis of [4Fe-4S] Proteins Using a Recombinant SUF System under Aerobic Conditions |
| Journal : | ACS Synthetic Biology |
| DOI : | 10.1021/acssynbio.3c00155 |
| Affiliations : | 1 Department of Chemical Engineering and Materials Engineering, National Central University, Taoyuan 32001, Taiwan 2 Graduate Institute of Environmental Engineering, National Central University, Taoyuan 32001, Taiwan 3 Earth-Life Science Institute, Tokyo Institute of Technology, Tokyo 152-8550, Japan 4 School of Life Science and Technology, Tokyo Institute of Technology, Tokyo 152-8550, Japan 5 Blue Marble Space Institute of Science, Seattle, Washington 98154, United States 6 Graduate School of Media and Governance, Keio University, Fujisawa 252-0882, Japan |
- The genetics of temperature adaptation: how does life thrive in extreme conditions? | Life Science and Technology News
- Scientists discover potential key missing link protein bridging eukaryotes and prokaryotes | Life Science and Technology News
- New study shows how complex metabolism may have self-assembled from simple precursors | Life Science and Technology News
- Study discovers process that may have produced first organic molecules for life on Earth | Life Science and Technology News
- New study reveals life’s earliest evolution was more complicated than previously suspected | Life Science and Technology News
- ELSI Research Suggests Life Thrived on Earth 3.5 Billion Years Ago | Tokyo Tech News
- Shawn McGlynn - Bridging disciplines and continents | Research Stories | Research
- Kosuke Fujishima | Researcher Finder - Tokyo Tech STAR Search
- Shawn Mcglynn | Researcher Finder - Tokyo Tech STAR Search
- Fujishima, Kosuke | Earth-Life Science Institute (ELSI)
- McGlynn, Shawn | Earth-Life Science Institute (ELSI)
- Fujishima Lab
- Shawn McGlynn Website
- Earth-Life Science Institute (ELSI)
- Department of Life Science and Technology, School of Life Science and Technology
- Department of Chemical and Materials Engineering, National Central University
- Graduate Institute of Environmental Engineering, National Central University
- Blue Marble Space Institute of Science
- Graduate School of Media and Governance, Keio University
- Latest Research News
Further Information
Kosuke Fujishima
Principal Investigator (Associate Professor)
Earth-Life Science Institute (ELSI),
Tokyo Institute of Technology
E-mail fuji@elsi.jp
Tel +81-3-5734-2126