Life Science and Technology News
Inflammation in regeneration: a friend or foe?
Scientists at Tokyo Institute of Technology have discovered a novel mechanism linking inflammation and organ regeneration in fish, which can be conserved among vertebrates.
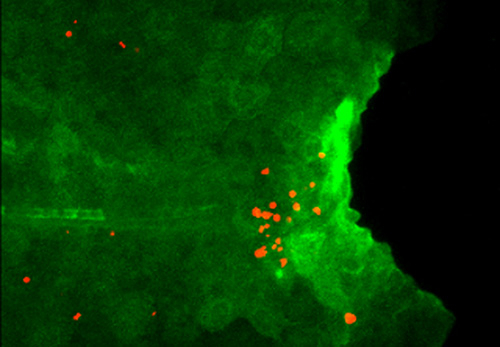
Figure 1. Excess Il1b induces apoptosis of regenerative cells.
Expression of Il1b (green) and apoptosis of regenerative cells (red) are detected in larval tail of the mutant which lacks macrophage. Il1b expression is visualized by using the transgenic zebrafish.
Regeneration is an inherent property of life. However, the potential to regenerate differs across species: while fish and amphibians can re-grow appendages such as limbs, tails, and fins, mammals, including humans, cannot restore injured organs to their original shape and function. Therefore, elucidation of molecular mechanisms underlying the amazing regenerative capacity of lower vertebrates can show approaches to restore complex organs in humans, which is a clinical goal of the future.
An international team of scientists led by Associate Professor Atsushi Kawakami, who teaches Life Science and Technology, from Tokyo Institute of Technology have disclosed a mechanism regulating regeneration of the caudal fin in zebrafish. To identify key molecules responsible for tissue repair, they compared gene transcription in the larvae of the wild-type and mutant zebrafish deficient in fin regeneration. They found that some inflammatory mediators, especially cytokine interleukin 1 beta (Il1b), were upregulated in the mutant and remained there for a long time after amputation of the larval tail. The mutant zebrafish also lacked myeloid cells such as macrophages, necessary to prevent programmed cell death (apoptosis) of the regenerative cells. The scientists therefore suspected a link among the increase in Il1b, absence of macrophages, and death of regenerative cells.
Il1b is considered to be mostly produced by myeloid cells. Surprisingly, after fin amputation, Il1b was primarily observed in epithelial cells surrounding the site of injury where it caused inflammation and apoptosis of the regenerative cells and inhibited the extension of the fin fold. However, if macrophages come to action, they could suppress Il1b expression, attenuate inflammation, and promote survival of the regenerative cells in the fin, thus behaving as critical regulators of inflammation during tissue repair.
All these data point to the negative effect of Il1b on the regenerative processes taking place after fin amputation. Yet, it is not that simple. By creating an Il1b -deficient zebrafish, the researchers found that transient, contrary to prolonged, presence of Il1b activated the expression of regeneration-induced genes and was essential for cell proliferation at the amputation site and regeneration of the injured fin.
Thus, the study of Dr. Kawakami and his colleagues revealed an unexpected association between regeneration and inflammation which acts as a double-edge sword: while acute inflammation is necessary to initiate tissue repair, chronic inflammation blocks further regeneration (Figure 2). As Il1b is evolutionary conserved in vertebrates, it remains to be determined whether similar mechanisms can function in mammals, including humans, as well as to identify anti-inflammatory factors released by macrophages.
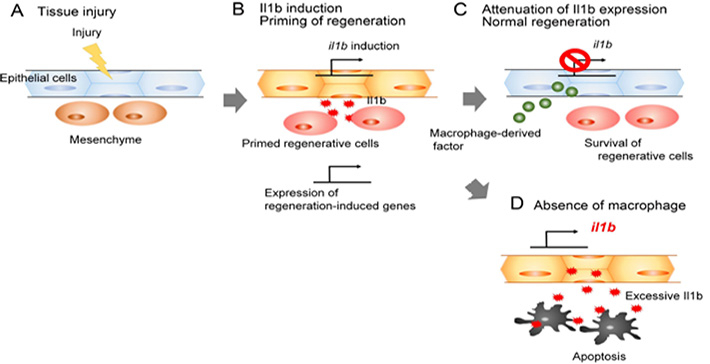
Figure 2. The role of Il1b and macrophages during fin regeneration.
After tissue injury (A), epithelial cells secrete Il1b which activates regeneration-induced genes and promotes proliferation of surrounding cells (B). Macrophages attenuate Il1b expression, preventing chronic inflammation (C); otherwise, regenerative cells die by apoptosis induced by Il1b excess (D).
Reference
| Authors: | Tomoya Hasegawa1, Christopher J. Hall2, Philip S. Crosier2, Gembu Abe3, Koichi Kawakami3, Akira Kudo1, Atsushi Kawakami1 |
|---|---|
| Title of original paper: | Transient inflammatory response mediated by interleukin-1β is required for proper regeneration in zebrafish fin fold |
| Journal: | eLife |
| DOI : | 10.7554/eLife.22716 |
| Affiliations : |
1 School of Bioscience and Biotechnology, Tokyo Institute of Technology 2 Department of Molecular Medicine and Pathology, School of Medical Sciences, University of Auckland 3 Division of Molecular and Developmental Biology, National Institute of Genetics, and Department of Genetics, SOKENDAI (The Graduate University for Advanced Studies) |
- Fibroblast growth factor signalling controls fin regeneration in zebrafish | Life Science and Technology News
- Kawakami Laboratory (Japanese)
- Researcher Profile | Tokyo Tech STAR Search - Atsushi Kawakami
- Labs spotlight #20 - Kawakami Laboratory - | Life Science and Technology News
- Latest Research News
School of Life Science and Technology
—Unravel the Complex and Diverse Phenomena of Life—
Information on School of Life Science and Technology inaugurated in April 2016
Further information
Associate Professor Atsushi Kawakami
School of Life Science and Technology
Email atkawaka@bio.titech.ac.jp
Tel +81-45-924-5717