Life Science and Technology News
【Labs spotlight】 Kobayashi Laboratory(until Mar. 2019)
Synthesis of Biologically Active Compounds
The Department has a variety of laboratories for Life Science and Technology, in which cutting-edge innovative research is being undertaken not only in basic science and engineering but also in the areas of medicine, pharmacy, agriculture, and multidisciplinary sciences.
This "Spotlight" series features a laboratory from the Department and introduces you to the laboratory's research projects and outcomes. This time we focus on Kobayashi Laboratory.
※Professor Kobayashi was retired on March 31, 2019.

Life Science and Technology
Professor Yuichi Kobayashi![]()
| Degree | PhD 1981, Tokyo Institute of Technology |
|---|---|
| Areas of Research | Synthetic organic chemistry, Medicinal chemistry, Drug design |
| Keywords | Organic synthesis, Natural products, Pd-catalyzed reaction, C-C bond formation |
Research interest
We are synthetic organic chemists and interested in synthesis of biologically important natural products that are hardly available from the natural sources. Synthetic design of targeted natural products are important step to achieve synthesis. We are also interested in carbon-carbon bond forming reactions using transition metals as reactants or catalysts. Furthermore, we have contributed to joint-research with biochemists using our compounds.
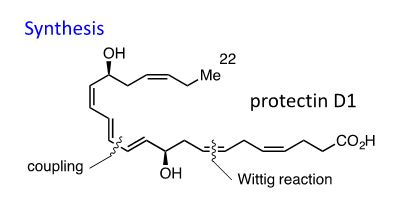
(1) Synthesis of Resolvins
Resolvins are metabolites of eicosatetraenoic acid (EPA) and docosahexaenoic acid (DHA) that are potent mediators of anti-inflammatory activity. We use several asymmetric and stereoselective reactions to construct the structure. High selectivity and yields are advantage of our syntheses.
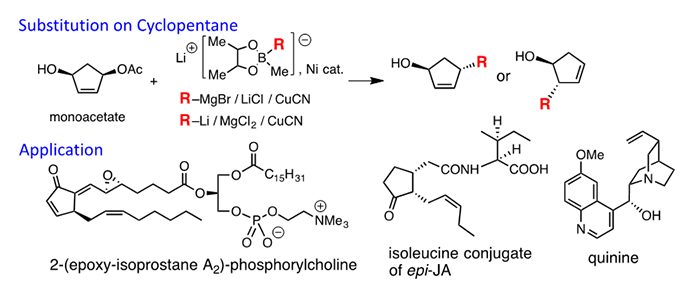
(2) Synthesis of Cyclopentanoids
We have interested in the use of the mono-acetate of cyclopentene-1,3-diol as the starting compound, which are available as optically active compounds. We have developed several reagents that react at the α carbon bearing OAc or at the γ carbon (olefin end). We have used this reaction to synthesize 2-(epoxy-isoprostane)-phosphorylcholines isolated from atherosclerotic lesion and epi-jasmonates including isoleucin conjugate of epi-jasmonic acid. Furthermore, the cyclopentene ring was converted to the piperidine ring in the synthesis of quinine and quinidine.
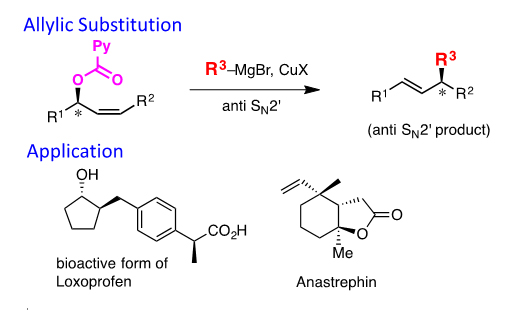
(3) Allylic substitution using the Picolinoxy Leaving Group
We found the picolinoxy group is the highly reactive leaving group in the allylic substitution of secondary allylic esters with organocopper reagents. This leaving group allows the use of less reactive aryl, hetero-aryl, and alkynyl copper reagents. Furthermore, this reaction is extended to construct quaternary carbons.
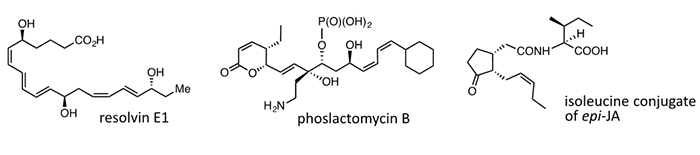
(4) Collaboration
We have successfully collaborated with compounds shown below.
Research findings
Selected publications
- [1] Asymmetric synthesis of 12-hydroxyheptadecatrienoic acid and its 5,6-dihydro- and 14,15-dehydro-derivatives. Y. Kobayashi, M. Morita, N. Ogawa, D. Kondo, T. Tojo, Org. Biomol. Chem. 2016, 14, 10667-10673.
- [2] Synthesis of (-)-piperitylmagnolol featuring ortho-selective deiodination and Pd-catalyzed allylation. A. Ikoma, N. Ogawa, D. Kondo, H. Kawada, Y. Kobayashi, Org. Lett. 2016, 18, 2074-2077.
- [3] Metal catalyst-free substitution of allylic and propargylic phosphates with diarylmethyl anions. H. Kawashima, N. Ogawa, R. Saeki, Y. Kobayashi, Chem. Commun. 2016, 52, 4918-4921.
- [4] Activation of marginally reactive boron enolates by MeLi for the formation of enol phosphates and synthesis of the Δ9-THC intermediate. H. Kawada, A. Ikoma, N. Ogawa, Y. Kobayashi, J. Org. Chem. 2015, 80, 9192-9199.
- [5] Synthesis of (-)-mesembrine using the quaternary carbon-constructing allylic substitution. T. Ozaki, Y. Kobayashi, Org. Chem. Front. 2015, 2, 328-335.
- [6] Synthesis of the PMB ether of 5,6-epoxyisoprostane E2 through aldol reaction of the α-bromocyclopentanone. H. Kawashima, Y. Kobayashi, Org. Lett. 2014, 16, 2598-2601.
- [7] Synthesis of maresin 1 and (7S)-isomer. N. Ogawa, T. Tojo, Y. Kobayashi, Tetrahedron Lett. 2014, 55, 2738-2741.
- [8] Synthesis of trans-2,6-disubstituted cyclohexanones through allylic substitution. Y. Kobayashi, C. Feng, A. Ikoma, N. Ogawa, T. Hirotsu, Org. Lett. 2014, 16, 760-763.
Selected by Synfacts 2014, 10(5), 0519 - [9] Synthesis of cyclobakuchiols A, B, and C using conformation-controlled stereoselective reactions. H. Kawashima, Y. Kaneko, M. Sakai, Y. Kobayashi, Chem. Eur. J. 2014, 20, 272-278.
- [10] Allylic substitution for construction of a chiral quaternary carbon possessing an aryl group. C. Feng, Y. Kobayashi, J. Org. Chem. 2013, 78, 3755-3766.
- [11] Synthesis of the amino acid conjugates of epi-jasmonic acid. N. Ogawa, Y. Kobayashi, Amino Acids, 2012, 42, 1955-1966.
- [12] Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. L. B. Sheard, X. Tan, H. Mao, J. Withers, G. Ben-Nissan, T. R. Hinds, Y. Kobayashi, F.-F. Hsu, M. Sharon, J. Browse, S. Y. He, J. Rizo, G. A. Howe, N. Zheng, Nature, 2010, 468, 400-405.